+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6ic1 | ||||||
|---|---|---|---|---|---|---|---|
| Title | urate oxidase under 90 bar of krypton | ||||||
 Components Components | Uricase | ||||||
 Keywords Keywords | OXIDOREDUCTASE / aspergillus flavus / homotetramer / purine metabolism / krypton / high pressure | ||||||
| Function / homology |  Function and homology information Function and homology informationurate oxidase activity / factor-independent urate hydroxylase / purine nucleobase catabolic process / urate catabolic process / peroxisome Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 1.1 Å FOURIER SYNTHESIS / Resolution: 1.1 Å | ||||||
 Authors Authors | Prange, T. / Colloc'h, N. / Carpentier, P. | ||||||
 Citation Citation |  Journal: Acta Cryst. D / Year: 2022 Journal: Acta Cryst. D / Year: 2022Title: Comparative study of the effects of high hydrostatic pressure per se and high argon pressure on urate oxidase ligand stabilization Authors: Prange, T. / Carpentier, P. / Dhaussy, A.C. / Girard, E. / Colloc'h, N. #1:  Journal: Current trends in X-ray crystallography / Year: 2011 Journal: Current trends in X-ray crystallography / Year: 2011Title: Current trends in X-ray Crystallography. Intech Open books Authors: Colloc'h, N. / Marassio, G. / Prange, T. #2:  Journal: Journal of Applied Crystallography / Year: 2016 Journal: Journal of Applied Crystallography / Year: 2016Title: Gas-sensitive biological crystals processed in pressurized oxygen and krypton atmospheres: deciphering gas channels in proteins using a novel soak-and-freeze methodology Authors: Lafumat, B. / Mueller-Dieckmann, C. / Leonard, G. / Colloc'h, N. / Prange, T. / Giraud, T. / Dobias, F. / Royant, A. / van der Linden, P. / Carpentier, P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6ic1.cif.gz 6ic1.cif.gz | 165.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6ic1.ent.gz pdb6ic1.ent.gz | 129.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6ic1.json.gz 6ic1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ic/6ic1 https://data.pdbj.org/pub/pdb/validation_reports/ic/6ic1 ftp://data.pdbj.org/pub/pdb/validation_reports/ic/6ic1 ftp://data.pdbj.org/pub/pdb/validation_reports/ic/6ic1 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6rgmC  1r51S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 34183.590 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: The six last residues are missing in the electron density Source: (gene. exp.)   References: UniProt: Q00511, factor-independent urate hydroxylase |
|---|
-Non-polymers , 6 types, 552 molecules 
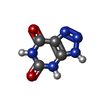









| #2: Chemical | ChemComp-MPD / ( | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-AZA / | ||||||
| #4: Chemical | | #5: Chemical | #6: Chemical | ChemComp-NA / | #7: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.96 Å3/Da / Density % sol: 57.8 % / Description: LARGE COLORLESS PRISMATIC CRYSTALS |
|---|---|
| Crystal grow | Temperature: 291 K / Method: batch mode / pH: 8 Details: 20 MicroL URATE OXIDASE (at 20 mg/ML+0.2MG/ML 8-AZAXANTHINE, 50mM TRIS) MIXED WIH 20 microL (TRIS + PEG 8000 4-8%). |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM30A / Wavelength: 0.999 Å / Beamline: BM30A / Wavelength: 0.999 Å |
| Detector | Type: DECTRIS PILATUS3 S 6M / Detector: PIXEL / Date: Jun 14, 2017 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.999 Å / Relative weight: 1 |
| Reflection | Resolution: 1.1→70.15 Å / Num. obs: 147453 / % possible obs: 93.1 % / Redundancy: 4.7 % / CC1/2: 0.999 / Rmerge(I) obs: 0.056 / Rpim(I) all: 0.028 / Rrim(I) all: 0.063 / Net I/σ(I): 10.1 |
| Reflection shell | Resolution: 1.1→1.16 Å / Redundancy: 4.8 % / Rmerge(I) obs: 0.46 / Mean I/σ(I) obs: 2.4 / Num. unique obs: 20666 / CC1/2: 0.879 / Rpim(I) all: 0.231 / Rrim(I) all: 0.516 / % possible all: 90 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: 1R51 Resolution: 1.1→70.15 Å / Cor.coef. Fo:Fc: 0.984 / SU B: 0.698 / SU ML: 0.014 / Cross valid method: NONE / ESU R: 0.021 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS - ANISOTROPIC REFINEMENT - NO RFREE USED (MEANINGLESS AT ATOMIC RESOLUTION)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15.938 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.1→70.15 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


















 PDBj
PDBj




