[English] 日本語
 Yorodumi
Yorodumi- PDB-6gt0: Nitrite-bound copper nitrite reductase from Achromobacter cyclocl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6gt0 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Nitrite-bound copper nitrite reductase from Achromobacter cycloclastes determined by serial femtosecond rotation crystallography | ||||||||||||
 Components Components | Copper-containing nitrite reductase | ||||||||||||
 Keywords Keywords | OXIDOREDUCTASE / FEMTOSECOND / SF-ROX / NITRITE REDUCTASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationdenitrification pathway / nitrite reductase (NO-forming) / nitrite reductase (NO-forming) activity / nitrate assimilation / periplasmic space / copper ion binding Similarity search - Function | ||||||||||||
| Biological species |  Achromobacter cycloclastes (bacteria) Achromobacter cycloclastes (bacteria) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FREE ELECTRON LASER / Resolution: 1.5 Å FREE ELECTRON LASER / Resolution: 1.5 Å | ||||||||||||
 Authors Authors | Halsted, T.P. / Yamashita, K. / Gopalasingam, C.C. / Shenoy, R.T. / Hirata, K. / Ago, H. / Ueno, G. / Eady, R.R. / Antonyuk, S.V. / Yamamoto, M. / Hasnain, S.S. | ||||||||||||
| Funding support |  United Kingdom, 3items United Kingdom, 3items
| ||||||||||||
 Citation Citation |  Journal: Iucrj / Year: 2019 Journal: Iucrj / Year: 2019Title: Catalytically important damage-free structures of a copper nitrite reductase obtained by femtosecond X-ray laser and room-temperature neutron crystallography. Authors: Halsted, T.P. / Yamashita, K. / Gopalasingam, C.C. / Shenoy, R.T. / Hirata, K. / Ago, H. / Ueno, G. / Blakeley, M.P. / Eady, R.R. / Antonyuk, S.V. / Yamamoto, M. / Hasnain, S.S. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6gt0.cif.gz 6gt0.cif.gz | 94.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6gt0.ent.gz pdb6gt0.ent.gz | 67.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6gt0.json.gz 6gt0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gt/6gt0 https://data.pdbj.org/pub/pdb/validation_reports/gt/6gt0 ftp://data.pdbj.org/pub/pdb/validation_reports/gt/6gt0 ftp://data.pdbj.org/pub/pdb/validation_reports/gt/6gt0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6gsqC  6gt2C  6gtiC  6gtjC  6gtkC  6gtlC  6gtnC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 40819.172 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Achromobacter cycloclastes (bacteria) / Gene: nirK / Plasmid: pET-26b(+) / Production host: Achromobacter cycloclastes (bacteria) / Gene: nirK / Plasmid: pET-26b(+) / Production host:  |
|---|
-Non-polymers , 5 types, 402 molecules 
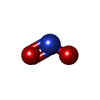







| #2: Chemical | | #3: Chemical | ChemComp-NO2 / | #4: Chemical | ChemComp-SO4 / | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.96 Å3/Da / Density % sol: 37.18 % |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: vapor diffusion, hanging drop / pH: 5 Details: 1.2 M ammonium sulphate, 100 mM citrate buffer, pH 5.0 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: Y |
|---|---|
| Diffraction source | Source:  FREE ELECTRON LASER / Site: FREE ELECTRON LASER / Site:  SACLA SACLA  / Beamline: BL2 / Wavelength: 1.242 Å / Beamline: BL2 / Wavelength: 1.242 Å |
| Detector | Type: RAYONIX MX225-HS / Detector: CCD / Date: Oct 5, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.242 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→54.86 Å / Num. obs: 45846 / % possible obs: 100 % / Redundancy: 154.7 % / Biso Wilson estimate: 22.6 Å2 / R split: 0.106 / Net I/σ(I): 6.6 |
| Reflection shell | Resolution: 1.5→1.54 Å / Redundancy: 50.8 % / Mean I/σ(I) obs: 2.3 / Num. unique obs: 2275 / R split: 0.853 / % possible all: 100 |
| Serial crystallography measurement | Pulse duration: 10 fsec. / Pulse energy: 437.7 µJ / XFEL pulse repetition rate: 30 Hz |
| Serial crystallography sample delivery | Method: fixed target |
| Serial crystallography sample delivery fixed target | Motion control: SACLA goniometer Sample holding: Mounted Hampton Cryo Loops 0.7 - 1.0 mm (HR4-965) Sample solvent: 3.4 M Na-malonate, pH 5.0 and 100 mM NaNO2. Support base: Mounted Hampton Cryo Loops 0.7 - 1.0 mm (HR4-965) |
| Serial crystallography data reduction | Crystal hits: 1257 / Frames indexed: 1039 / Frames total: 1257 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.5→54.86 Å / Cor.coef. Fo:Fc: 0.976 / Cor.coef. Fo:Fc free: 0.969 / SU B: 1.341 / SU ML: 0.049 / Cross valid method: THROUGHOUT / ESU R: 0.068 / ESU R Free: 0.069 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 19.23 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.5→54.86 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj




