[English] 日本語
 Yorodumi
Yorodumi- PDB-6g2d: Citrate-induced acetyl-CoA carboxylase (ACC-Cit) filament at 5.4 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6g2d | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
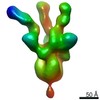
| Title | Citrate-induced acetyl-CoA carboxylase (ACC-Cit) filament at 5.4 A resolution | ||||||||||||
 Components Components | Acetyl-CoA carboxylase 1 | ||||||||||||
 Keywords Keywords | LIGASE / Filament / Helical / Multienzyme / Biotin-dependent carboxylase | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationacetyl-CoA carboxylase / fatty-acyl-CoA biosynthetic process / Defective HLCS causes multiple carboxylase deficiency / Biotin transport and metabolism / malonyl-CoA biosynthetic process / Fatty acyl-CoA biosynthesis / ChREBP activates metabolic gene expression / acetyl-CoA carboxylase activity / acetyl-CoA metabolic process / tissue homeostasis ...acetyl-CoA carboxylase / fatty-acyl-CoA biosynthetic process / Defective HLCS causes multiple carboxylase deficiency / Biotin transport and metabolism / malonyl-CoA biosynthetic process / Fatty acyl-CoA biosynthesis / ChREBP activates metabolic gene expression / acetyl-CoA carboxylase activity / acetyl-CoA metabolic process / tissue homeostasis / Carnitine shuttle / lipid homeostasis / Activation of gene expression by SREBF (SREBP) / fibrillar center / fatty acid biosynthetic process / cellular response to prostaglandin E stimulus / actin cytoskeleton / protein homotetramerization / mitochondrion / ATP binding / metal ion binding / identical protein binding / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 5.4 Å | ||||||||||||
 Authors Authors | Hunkeler, M. / Hagmann, A. / Stuttfeld, E. / Chami, M. / Stahlberg, H. / Maier, T. | ||||||||||||
| Funding support |  Switzerland, 3items Switzerland, 3items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Structural basis for regulation of human acetyl-CoA carboxylase. Authors: Moritz Hunkeler / Anna Hagmann / Edward Stuttfeld / Mohamed Chami / Yakir Guri / Henning Stahlberg / Timm Maier /   Abstract: Acetyl-CoA carboxylase catalyses the ATP-dependent carboxylation of acetyl-CoA, a rate-limiting step in fatty acid biosynthesis. Eukaryotic acetyl-CoA carboxylases are large, homodimeric multienzymes. ...Acetyl-CoA carboxylase catalyses the ATP-dependent carboxylation of acetyl-CoA, a rate-limiting step in fatty acid biosynthesis. Eukaryotic acetyl-CoA carboxylases are large, homodimeric multienzymes. Human acetyl-CoA carboxylase occurs in two isoforms: the metabolic, cytosolic ACC1, and ACC2, which is anchored to the outer mitochondrial membrane and controls fatty acid β-oxidation. ACC1 is regulated by a complex interplay of phosphorylation, binding of allosteric regulators and protein-protein interactions, which is further linked to filament formation. These filaments were discovered in vitro and in vivo 50 years ago, but the structural basis of ACC1 polymerization and regulation remains unknown. Here, we identify distinct activated and inhibited ACC1 filament forms. We obtained cryo-electron microscopy structures of an activated filament that is allosterically induced by citrate (ACC-citrate), and an inactivated filament form that results from binding of the BRCT domains of the breast cancer type 1 susceptibility protein (BRCA1). While non-polymeric ACC1 is highly dynamic, filament formation locks ACC1 into different catalytically competent or incompetent conformational states. This unique mechanism of enzyme regulation via large-scale conformational changes observed in ACC1 has potential uses in engineering of switchable biosynthetic systems. Dissecting the regulation of acetyl-CoA carboxylase opens new paths towards counteracting upregulation of fatty acid biosynthesis in disease. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6g2d.cif.gz 6g2d.cif.gz | 1.9 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6g2d.ent.gz pdb6g2d.ent.gz | 1.5 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6g2d.json.gz 6g2d.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/g2/6g2d https://data.pdbj.org/pub/pdb/validation_reports/g2/6g2d ftp://data.pdbj.org/pub/pdb/validation_reports/g2/6g2d ftp://data.pdbj.org/pub/pdb/validation_reports/g2/6g2d | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4342MC  4343C  4344C  6g2hC  6g2iC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 272632.844 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ACACA, ACAC, ACC1, ACCA / Production host: Homo sapiens (human) / Gene: ACACA, ACAC, ACC1, ACCA / Production host:  References: UniProt: Q13085, acetyl-CoA carboxylase, biotin carboxylase |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: FILAMENT / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Multienzyme / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 40 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) Details: Data was collected in movie mode with a total of 40 e-/A2 over a total of 40 frames. Frames 1-30 were used for final reconstruction. |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: RELION / Version: 2.1b1 / Category: 3D reconstruction | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 5.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 131062 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Stereochemistry target values: GeoStd + Monomer Library Details: The clash score value (3.5) reported in the publication associated with this model was obtained directly from phenix.real_space_refine version 1.11.1-2575. We acknowledge that the current ...Details: The clash score value (3.5) reported in the publication associated with this model was obtained directly from phenix.real_space_refine version 1.11.1-2575. We acknowledge that the current validation toolchain of wwwPDB produces slightly higher values, possibly linked to the handling of cif files (required as this submission contains more than 100.000 atoms) and protons and have locally reproduced this difference by using earlier software versions. | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller






 PDBj
PDBj






