[English] 日本語
 Yorodumi
Yorodumi- PDB-6cec: Crystal structure of fragment 3-(3-Methoxy-2-quinoxalinyl)propano... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6cec | ||||||
|---|---|---|---|---|---|---|---|
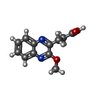
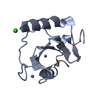
| Title | Crystal structure of fragment 3-(3-Methoxy-2-quinoxalinyl)propanoic acid bound in the ubiquitin binding pocket of the HDAC6 zinc-finger domain | ||||||
 Components Components | Histone deacetylase 6 | ||||||
 Keywords Keywords | HYDROLASE / HISTONE DEACETYLASE / HDAC / HDAC6 / UBIQUITIN / STRUCTURAL GENOMICS CONSORTIUM / SGC | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of hydrogen peroxide metabolic process / cellular response to topologically incorrect protein / polyubiquitinated misfolded protein transport / positive regulation of cellular response to oxidative stress / negative regulation of aggrephagy / positive regulation of cholangiocyte proliferation / response to misfolded protein / positive regulation of protein oligomerization / negative regulation of axon extension involved in axon guidance / type 2 mitophagy ...negative regulation of hydrogen peroxide metabolic process / cellular response to topologically incorrect protein / polyubiquitinated misfolded protein transport / positive regulation of cellular response to oxidative stress / negative regulation of aggrephagy / positive regulation of cholangiocyte proliferation / response to misfolded protein / positive regulation of protein oligomerization / negative regulation of axon extension involved in axon guidance / type 2 mitophagy / positive regulation of RIG-I signaling pathway / negative regulation of protein-containing complex disassembly / peroxidase inhibitor activity / erythrocyte enucleation / regulation of autophagy of mitochondrion / Cilium Assembly / tubulin deacetylation / protein-containing complex disassembly / regulation of establishment of protein localization / collateral sprouting / Transcriptional regulation by RUNX2 / tubulin deacetylase activity / negative regulation of microtubule depolymerization / lysosome localization / ATPase inhibitor activity / cilium disassembly / histone deacetylase activity, hydrolytic mechanism / misfolded protein binding / ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway / dendritic spine morphogenesis / positive regulation of type 2 mitophagy / protein deacetylation / aggresome assembly / positive regulation of dendrite morphogenesis / regulation of androgen receptor signaling pathway / regulation of mitochondrion organization / Transferases; Acyltransferases; Aminoacyltransferases / cellular response to misfolded protein / regulation of fat cell differentiation / protein lysine deacetylase activity / Hydrolases; Acting on carbon-nitrogen bonds, other than peptide bonds; In linear amides / histone deacetylase activity / aggresome / microtubule associated complex / cellular response to parathyroid hormone stimulus / positive regulation of intracellular estrogen receptor signaling pathway / response to dexamethasone / response to corticosterone / Notch-HLH transcription pathway / axonal transport of mitochondrion / negative regulation of gene expression, epigenetic / regulation of microtubule-based movement / RUNX2 regulates osteoblast differentiation / histone deacetylase complex / response to immobilization stress / cell leading edge / dynein complex binding / protein quality control for misfolded or incompletely synthesized proteins / positive regulation of epithelial cell migration / polyubiquitin modification-dependent protein binding / cilium assembly / regulation of macroautophagy / HSF1 activation / positive regulation of synaptic transmission, glutamatergic / alpha-tubulin binding / beta-tubulin binding / negative regulation of protein-containing complex assembly / negative regulation of proteolysis / multivesicular body / inclusion body / axon cytoplasm / antiviral innate immune response / epigenetic regulation of gene expression / actin filament organization / ubiquitin binding / transcription corepressor binding / response to amphetamine / intracellular protein transport / Hsp90 protein binding / regulation of protein stability / Late endosomal microautophagy / beta-catenin binding / caveola / regulation of autophagy / NOTCH1 Intracellular Domain Regulates Transcription / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants / protein destabilization / tau protein binding / epidermal growth factor receptor signaling pathway / cellular response to hydrogen peroxide / histone deacetylase binding / protein polyubiquitination / Chaperone Mediated Autophagy / Aggrephagy / cellular response to heat / actin binding / microtubule binding / microtubule / perikaryon Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.55 Å X-RAY DIFFRACTION / Resolution: 1.55 Å | ||||||
 Authors Authors | Harding, R.J. / Halabelian, L. / Ferreira de Freitas, R. / Franzoni, I. / Ravichandran, M. / Lautens, M. / Santhakumar, V. / Schapira, M. / Bountra, C. / Edwards, A.M. ...Harding, R.J. / Halabelian, L. / Ferreira de Freitas, R. / Franzoni, I. / Ravichandran, M. / Lautens, M. / Santhakumar, V. / Schapira, M. / Bountra, C. / Edwards, A.M. / Arrowsmith, C.M. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: J. Med. Chem. / Year: 2018 Journal: J. Med. Chem. / Year: 2018Title: Identification and Structure-Activity Relationship of HDAC6 Zinc-Finger Ubiquitin Binding Domain Inhibitors. Authors: Ferreira de Freitas, R. / Harding, R.J. / Franzoni, I. / Ravichandran, M. / Mann, M.K. / Ouyang, H. / Lautens, M. / Santhakumar, V. / Arrowsmith, C.H. / Schapira, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6cec.cif.gz 6cec.cif.gz | 38.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6cec.ent.gz pdb6cec.ent.gz | 23.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6cec.json.gz 6cec.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ce/6cec https://data.pdbj.org/pub/pdb/validation_reports/ce/6cec ftp://data.pdbj.org/pub/pdb/validation_reports/ce/6cec ftp://data.pdbj.org/pub/pdb/validation_reports/ce/6cec | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6ce6C  6ce8C  6ceaC  6cedC  6ceeC  6cefC  5kh3S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 11932.607 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HDAC6, KIAA0901, JM21 / Plasmid: pET28-lic / Production host: Homo sapiens (human) / Gene: HDAC6, KIAA0901, JM21 / Plasmid: pET28-lic / Production host:  | ||||||
|---|---|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | ChemComp-UNX / #4: Chemical | ChemComp-EY7 / | #5: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.09 Å3/Da / Density % sol: 41.07 % / Mosaicity: 0 ° |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 4.6 Details: 2 M Na-formate, 0.2 M Na-acetate pH4.6, 5 % ethylene glycol |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.54178 Å ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.54178 Å | ||||||||||||||||||||||||||||||
| Detector | Type: RIGAKU SATURN A200 / Detector: CCD / Date: Feb 6, 2018 | ||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.54178 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.55→32.92 Å / Num. obs: 15017 / % possible obs: 99.6 % / Redundancy: 6.7 % / CC1/2: 1 / Rmerge(I) obs: 0.036 / Rpim(I) all: 0.015 / Rrim(I) all: 0.039 / Net I/σ(I): 29.3 / Num. measured all: 100667 / Scaling rejects: 1 | ||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Starting model: pdbid 5KH3 Resolution: 1.55→32.9 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.959 / SU B: 1.232 / SU ML: 0.045 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.074 / ESU R Free: 0.073 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: Users of this crystal structure: verify our intepretion of the electron density. Amplitudes and unmerged intensities are included with this deposition. Diffraction images will be deposited ...Details: Users of this crystal structure: verify our intepretion of the electron density. Amplitudes and unmerged intensities are included with this deposition. Diffraction images will be deposited at a later date in a public repository. Geometry restraints for the ligand were prepared with GRADE.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 40.42 Å2 / Biso mean: 15.839 Å2 / Biso min: 8.72 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.55→32.9 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.55→1.59 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller










 PDBj
PDBj