[English] 日本語
 Yorodumi
Yorodumi- PDB-6bxk: Crystal structure of Pyrococcus horikoshii Dph2 with 4Fe-4S clust... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6bxk | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Pyrococcus horikoshii Dph2 with 4Fe-4S cluster and MTA | ||||||
 Components Components | 2-(3-amino-3-carboxypropyl)histidine synthase | ||||||
 Keywords Keywords | BIOSYNTHETIC PROTEIN / DIPHTHAMIDE BIOSYNTHESIS / RADICAL SAM ENZYME | ||||||
| Function / homology |  Function and homology information Function and homology informationS-adenosylmethionine catabolic process / 2-(3-amino-3-carboxypropyl)histidine synthase / 2-(3-amino-3-carboxypropyl)histidine synthase activity / protein histidyl modification to diphthamide / transferase activity, transferring alkyl or aryl (other than methyl) groups / 4 iron, 4 sulfur cluster binding / metal ion binding Similarity search - Function | ||||||
| Biological species |   Pyrococcus horikoshii (archaea) Pyrococcus horikoshii (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.347 Å FOURIER SYNTHESIS / Resolution: 2.347 Å | ||||||
 Authors Authors | Torelli, A.T. / Fenwick, M.K. / Zhang, Y. / Dong, M. / Kathiresan, V. / Carantoa, J.D. / Dzikovski, B. / Lancaster, K.M. / Freed, J.H. / Hoffman, B.M. ...Torelli, A.T. / Fenwick, M.K. / Zhang, Y. / Dong, M. / Kathiresan, V. / Carantoa, J.D. / Dzikovski, B. / Lancaster, K.M. / Freed, J.H. / Hoffman, B.M. / Lin, H. / Ealick, S.E. | ||||||
 Citation Citation |  Journal: Science / Year: 2018 Journal: Science / Year: 2018Title: Organometallic and radical intermediates reveal mechanism of diphthamide biosynthesis. Authors: Dong, M. / Kathiresan, V. / Fenwick, M.K. / Torelli, A.T. / Zhang, Y. / Caranto, J.D. / Dzikovski, B. / Sharma, A. / Lancaster, K.M. / Freed, J.H. / Ealick, S.E. / Hoffman, B.M. / Lin, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6bxk.cif.gz 6bxk.cif.gz | 281.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6bxk.ent.gz pdb6bxk.ent.gz | 225 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6bxk.json.gz 6bxk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6bxk_validation.pdf.gz 6bxk_validation.pdf.gz | 943 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6bxk_full_validation.pdf.gz 6bxk_full_validation.pdf.gz | 948.2 KB | Display | |
| Data in XML |  6bxk_validation.xml.gz 6bxk_validation.xml.gz | 25.2 KB | Display | |
| Data in CIF |  6bxk_validation.cif.gz 6bxk_validation.cif.gz | 34.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bx/6bxk https://data.pdbj.org/pub/pdb/validation_reports/bx/6bxk ftp://data.pdbj.org/pub/pdb/validation_reports/bx/6bxk ftp://data.pdbj.org/pub/pdb/validation_reports/bx/6bxk | HTTPS FTP |
-Related structure data
| Related structure data |  6bxlC  6bxmC  6bxnC  6bxoC  3lzcS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
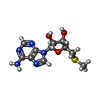
| #1: Protein | Mass: 42538.289 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pyrococcus horikoshii (strain ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3) (archaea) Pyrococcus horikoshii (strain ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3) (archaea)Strain: ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3 Gene: dph2, PH1105 / Production host:  References: UniProt: O58832, 2-(3-amino-3-carboxypropyl)histidine synthase #2: Chemical | #3: Chemical | #4: Chemical | #5: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.13 Å3/Da / Density % sol: 42.12 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop Details: 31-35% (v/v) PEG 400, either 0.1 M sodium citrate buffer pH 5.5 or MES buffer pH 6.5, 0.2 M Li2SO4 and 2% (v/v) ethylene glycol |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.9792 Å / Beamline: 24-ID-C / Wavelength: 0.9792 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jun 1, 2009 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9792 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.35→50 Å / Num. obs: 29510 / % possible obs: 94.8 % / Redundancy: 4.4 % / Biso Wilson estimate: 53.56 Å2 / Rmerge(I) obs: 0.062 / Χ2: 1.031 / Net I/σ(I): 12 / Num. measured all: 130240 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: 3LZC Resolution: 2.347→40.48 Å / SU ML: 0.35 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 31.79
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.347→40.48 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj