[English] 日本語
 Yorodumi
Yorodumi- PDB-5yts: Crystal structure of YB1 cold-shock domain in complex with UCUUCU -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5yts | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of YB1 cold-shock domain in complex with UCUUCU | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | RNA BINDING PROTEIN/RNA / YB1 / cold-shock domain / CUUC / RNA BINDING PROTEIN / RNA BINDING PROTEIN-RNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationtRNA transport / CRD-mediated mRNA stability complex / C5-methylcytidine-containing RNA reader activity / miRNA transport / negative regulation of striated muscle cell differentiation / negative regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / Noncanonical activation of NOTCH3 / RNA transport / CRD-mediated mRNA stabilization / protein localization to cytoplasmic stress granule ...tRNA transport / CRD-mediated mRNA stability complex / C5-methylcytidine-containing RNA reader activity / miRNA transport / negative regulation of striated muscle cell differentiation / negative regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay / Noncanonical activation of NOTCH3 / RNA transport / CRD-mediated mRNA stabilization / protein localization to cytoplasmic stress granule / histone pre-mRNA 3'end processing complex / embryonic morphogenesis / U12-type spliceosomal complex / positive regulation of cytoplasmic translation / mRNA stabilization / cellular response to interleukin-7 / miRNA binding / mRNA Splicing - Minor Pathway / Processing of Capped Intron-Containing Pre-mRNA / positive regulation of cell division / negative regulation of cellular senescence / epidermis development / mRNA Splicing - Major Pathway / RNA splicing / P-body / SMAD2/SMAD3:SMAD4 heterotrimer regulates transcription / mRNA processing / Interferon gamma signaling / cytoplasmic stress granule / sequence-specific double-stranded DNA binding / single-stranded DNA binding / regulation of gene expression / GTPase binding / double-stranded DNA binding / in utero embryonic development / nucleic acid binding / negative regulation of translation / ribonucleoprotein complex / intracellular membrane-bounded organelle / mRNA binding / synapse / chromatin binding / regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / endoplasmic reticulum / positive regulation of transcription by RNA polymerase II / DNA binding / RNA binding / extracellular exosome / extracellular region / nucleoplasm / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.77 Å MOLECULAR REPLACEMENT / Resolution: 1.77 Å | |||||||||
 Authors Authors | Yang, X. / Huang, Y. | |||||||||
| Funding support |  China, 2items China, 2items
| |||||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2019 Journal: J.Biol.Chem. / Year: 2019Title: Crystal structure of a Y-box binding protein 1 (YB-1)-RNA complex reveals key features and residues interacting with RNA. Authors: Yang, X.J. / Zhu, H. / Mu, S.R. / Wei, W.J. / Yuan, X. / Wang, M. / Liu, Y. / Hui, J. / Huang, Y. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5yts.cif.gz 5yts.cif.gz | 33.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5yts.ent.gz pdb5yts.ent.gz | 20.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5yts.json.gz 5yts.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5yts_validation.pdf.gz 5yts_validation.pdf.gz | 454.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5yts_full_validation.pdf.gz 5yts_full_validation.pdf.gz | 454.9 KB | Display | |
| Data in XML |  5yts_validation.xml.gz 5yts_validation.xml.gz | 6.2 KB | Display | |
| Data in CIF |  5yts_validation.cif.gz 5yts_validation.cif.gz | 7.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yt/5yts https://data.pdbj.org/pub/pdb/validation_reports/yt/5yts ftp://data.pdbj.org/pub/pdb/validation_reports/yt/5yts ftp://data.pdbj.org/pub/pdb/validation_reports/yt/5yts | HTTPS FTP |
-Related structure data
| Related structure data |  5yttC  5ytvC  5ytxC  3pf5S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 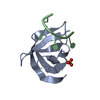
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 8997.026 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: YBX1, NSEP1, YB1 / Production host: Homo sapiens (human) / Gene: YBX1, NSEP1, YB1 / Production host:  |
|---|---|
| #2: RNA chain | Mass: 1790.069 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
| #3: Chemical | ChemComp-SO4 / |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.14 Å3/Da / Density % sol: 42.41 % |
|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 4.5 Details: 30 % (v/v) 2-methyl-2,4-pentanediol, 0.1 M sodium acetate, pH 4.5, 25 % (w/v) PEG 1500 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL17U1 / Wavelength: 0.979 Å / Beamline: BL17U1 / Wavelength: 0.979 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Dec 12, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979 Å / Relative weight: 1 |
| Reflection | Resolution: 1.77→50 Å / Num. obs: 16820 / % possible obs: 99 % / Redundancy: 7.3 % / Net I/σ(I): 30.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3PF5 Resolution: 1.77→30 Å / SU ML: 0.2 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 24.99
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.77→30 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj







