Entry Database : PDB / ID : 5upzTitle HIV-1 wild Type protease with GRL-0518A , an isophthalamide-derived P2-P3 ligand with the para-hydoxymethyl sulfonamide isostere as the P2' group Protease Keywords / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Method / / / Resolution : 1.27 Å Authors Wang, Y.-F. / Agniswamy, J. / Weber, I.T. Funding support Organization Grant number Country National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) GM53386 National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) GM62920 Department of Energy (DOE, United States) No. W-31-109-Eng-38 National Institutes of Health/National Cancer Institute (NIH/NCI) the Intramural Research Program of the Center for Cancer Research Ministry of Education, Culture, Sports, Science and Technology (Japan) a Grant-in-Aid for Scientific Research (Priority Areas) he Ministry of Health, Welfare, and Labor a Grant for Promotion of AIDS Research Ministry of Education, Culture, Sports, Science and Technology (Japan) the Grant to the Cooperative Research Project on Clinical and Epidemiological Studies of Emerging and Reemerging Infectious Diseases (Renkei Jigyo) Purdue University the Purdue University Center for Cancer Research
Journal : Bioorg. Med. Chem. / Year : 2017Title : Design of novel HIV-1 protease inhibitors incorporating isophthalamide-derived P2-P3 ligands: Synthesis, biological evaluation and X-ray structural studies of inhibitor-HIV-1 protease complex.Authors : Ghosh, A.K. / Brindisi, M. / Nyalapatla, P.R. / Takayama, J. / Ella-Menye, J.R. / Yashchuk, S. / Agniswamy, J. / Wang, Y.F. / Aoki, M. / Amano, M. / Weber, I.T. / Mitsuya, H. History Deposition Feb 5, 2017 Deposition site / Processing site Revision 1.0 May 10, 2017 Provider / Type Revision 1.1 Sep 27, 2017 Group / Category / Item Revision 1.2 Oct 4, 2017 Group / Category Item / _citation.page_first / _citation.page_lastRevision 1.3 Dec 4, 2019 Group / Category / Item Revision 1.4 Oct 4, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / diffrn_radiation_wavelength / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
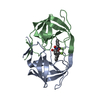
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 Human immunodeficiency virus 1
Human immunodeficiency virus 1 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.27 Å
MOLECULAR REPLACEMENT / Resolution: 1.27 Å  Authors
Authors United States,
United States,  Japan, 8items
Japan, 8items  Citation
Citation Journal: Bioorg. Med. Chem. / Year: 2017
Journal: Bioorg. Med. Chem. / Year: 2017 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5upz.cif.gz
5upz.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5upz.ent.gz
pdb5upz.ent.gz PDB format
PDB format 5upz.json.gz
5upz.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/up/5upz
https://data.pdbj.org/pub/pdb/validation_reports/up/5upz ftp://data.pdbj.org/pub/pdb/validation_reports/up/5upz
ftp://data.pdbj.org/pub/pdb/validation_reports/up/5upz

 Links
Links Assembly
Assembly
 Components
Components
 Human immunodeficiency virus 1 / Gene: pol / Plasmid: pET11a / Production host:
Human immunodeficiency virus 1 / Gene: pol / Plasmid: pET11a / Production host: 









 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 22-BM / Wavelength: 1 Å
/ Beamline: 22-BM / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller


















 PDBj
PDBj






