[English] 日本語
 Yorodumi
Yorodumi- PDB-6dj7: HIV-1 protease with mutation L76V in complex with GRL-5010 (gem-d... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6dj7 | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIV-1 protease with mutation L76V in complex with GRL-5010 (gem-difluoro-bis-tetrahydrofuran as P2 ligand) | ||||||
 Components Components | HIV-1 protease | ||||||
 Keywords Keywords | HYDROLASE / Antiviral agents / HIV drug resistance / ANTIVIRAL PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationintegrase activity / Integration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / Minus-strand DNA synthesis / Plus-strand DNA synthesis / Uncoating of the HIV Virion / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Early Phase of HIV Life Cycle / Integration of provirus ...integrase activity / Integration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / Minus-strand DNA synthesis / Plus-strand DNA synthesis / Uncoating of the HIV Virion / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Early Phase of HIV Life Cycle / Integration of provirus / APOBEC3G mediated resistance to HIV-1 infection / Binding and entry of HIV virion / viral life cycle / HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / Assembly Of The HIV Virion / protein processing / Budding and maturation of HIV virion / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / peptidase activity / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / identical protein binding Similarity search - Function | ||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.31 Å MOLECULAR REPLACEMENT / Resolution: 1.31 Å | ||||||
 Authors Authors | Wong-Sam, A.E. / Wang, Y.F. / Weber, I.T. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: ACS Omega / Year: 2018 Journal: ACS Omega / Year: 2018Title: Drug Resistance Mutation L76V Alters Nonpolar Interactions at the Flap-Core Interface of HIV-1 Protease. Authors: Wong-Sam, A. / Wang, Y.F. / Zhang, Y. / Ghosh, A.K. / Harrison, R.W. / Weber, I.T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6dj7.cif.gz 6dj7.cif.gz | 115.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6dj7.ent.gz pdb6dj7.ent.gz | 88 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6dj7.json.gz 6dj7.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dj/6dj7 https://data.pdbj.org/pub/pdb/validation_reports/dj/6dj7 ftp://data.pdbj.org/pub/pdb/validation_reports/dj/6dj7 ftp://data.pdbj.org/pub/pdb/validation_reports/dj/6dj7 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6difC  6dilC  6dj1C  6dj2C  6dj5C  3nu3S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 10726.650 Da / Num. of mol.: 2 / Mutation: Q7K, L33I, L63I, C67A, L76V, C95A Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Gene: pol / Production host: Human immunodeficiency virus 1 / Gene: pol / Production host:  |
|---|
-Non-polymers , 6 types, 198 molecules 


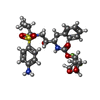







| #2: Chemical | ChemComp-NA / | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #3: Chemical | | #4: Chemical | #5: Chemical | ChemComp-G10 / ( | #6: Chemical | #7: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.7 Å3/Da / Density % sol: 54.48 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 5.2 Details: 1.15 M NaCl, 0.1 M NaOAc, pH 5.2 4.1 mg/ml protease stock, 1:5 protease to inhibitor 1:1 liquor to sample, soaked in 30% glycerol cryoprotectant PH range: 5.2 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 1 Å / Beamline: 22-ID / Wavelength: 1 Å |
| Detector | Type: RAYONIX MX300-HS / Detector: CCD / Date: Feb 28, 2016 |
| Radiation | Monochromator: Y / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.31→50 Å / Num. obs: 55212 / % possible obs: 96 % / Redundancy: 4.4 % / Rmerge(I) obs: 0.087 / Net I/σ(I): 13.3 |
| Reflection shell | Resolution: 1.31→1.36 Å / Redundancy: 1.8 % / Rmerge(I) obs: 0.454 / Mean I/σ(I) obs: 2.1 / % possible all: 77.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3NU3 Resolution: 1.31→32 Å / Cross valid method: FREE R-VALUE / σ(F): 0 Details: ANISOTROPIC REFINEMENT REDUCED FREE R (NO CUTOFF) BY ?
| |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 99 / Occupancy sum hydrogen: 0 / Occupancy sum non hydrogen: 1703.4 | |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.31→32 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





















 PDBj
PDBj












