+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5tes | ||||||
|---|---|---|---|---|---|---|---|
| Title | TEV Cleaved Human ATP Citrate Lyase Bound to Citrate and ADP | ||||||
 Components Components | (ATP-citrate synthase) x 2 | ||||||
 Keywords Keywords | TRANSFERASE / TEV cleaved / ATP-grasp fold / citrate binding | ||||||
| Function / homology |  Function and homology information Function and homology informationATP citrate synthase / ATP citrate synthase activity / citrate metabolic process / Fatty acyl-CoA biosynthesis / acetyl-CoA biosynthetic process / ChREBP activates metabolic gene expression / coenzyme A metabolic process / oxaloacetate metabolic process / negative regulation of ferroptosis / cholesterol biosynthetic process ...ATP citrate synthase / ATP citrate synthase activity / citrate metabolic process / Fatty acyl-CoA biosynthesis / acetyl-CoA biosynthetic process / ChREBP activates metabolic gene expression / coenzyme A metabolic process / oxaloacetate metabolic process / negative regulation of ferroptosis / cholesterol biosynthetic process / lipid biosynthetic process / fatty acid biosynthetic process / azurophil granule lumen / ficolin-1-rich granule lumen / ciliary basal body / Neutrophil degranulation / extracellular exosome / extracellular region / nucleoplasm / ATP binding / metal ion binding / membrane / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2.4 Å SYNCHROTRON / Resolution: 2.4 Å | ||||||
 Authors Authors | Hu, J. / Fraser, M.E. | ||||||
 Citation Citation |  Journal: Acta Crystallogr D Struct Biol / Year: 2017 Journal: Acta Crystallogr D Struct Biol / Year: 2017Title: Binding of hydroxycitrate to human ATP-citrate lyase. Authors: Hu, J. / Komakula, A. / Fraser, M.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5tes.cif.gz 5tes.cif.gz | 293.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5tes.ent.gz pdb5tes.ent.gz | 235.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5tes.json.gz 5tes.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/te/5tes https://data.pdbj.org/pub/pdb/validation_reports/te/5tes ftp://data.pdbj.org/pub/pdb/validation_reports/te/5tes ftp://data.pdbj.org/pub/pdb/validation_reports/te/5tes | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5tdeC  5tdfC  5tdmC  5tdzC  5te1C  5teqC  5tetC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 47836.633 Da / Num. of mol.: 1 / Fragment: unp residues 1-425 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ACLY / Production host: Homo sapiens (human) / Gene: ACLY / Production host:  |
|---|---|
| #2: Protein | Mass: 35421.570 Da / Num. of mol.: 1 / Fragment: unp residues 488-810 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ACLY / Production host: Homo sapiens (human) / Gene: ACLY / Production host:  |
-Non-polymers , 4 types, 247 molecules 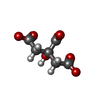






| #3: Chemical | ChemComp-FLC / | ||||
|---|---|---|---|---|---|
| #4: Chemical | | #5: Chemical | ChemComp-ADP / | #6: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|---|
| Sequence details | THE AMINO-TERMINAL PORTION OF HUMAN ACLY WAS REDESIGNED TO HAVE TEV PROTEASE CLEAVAGE SITES ...THE AMINO-TERMINAL PORTION OF HUMAN ACLY WAS REDESIGNED |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.86 Å3/Da / Density % sol: 56.97 % |
|---|---|
| Crystal grow | Temperature: 294 K / Method: vapor diffusion, hanging drop / pH: 8.8 Details: 12.5% P3350, 100 mM TrisHCl pH 8.8, 125 mM ammonium chloride |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CLSI CLSI  / Beamline: 08ID-1 / Wavelength: 0.97948 Å / Beamline: 08ID-1 / Wavelength: 0.97948 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Jul 17, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97948 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→49.75 Å / Num. obs: 37140 / % possible obs: 97.4 % / Redundancy: 3.7 % / Net I/σ(I): 7.1 |
- Processing
Processing
| Software | Name: PHENIX / Version: (1.10_2152: ???) / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.4→46.89 Å / SU ML: 0.34 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 25.2 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.4→46.89 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj









