[English] 日本語
 Yorodumi
Yorodumi- PDB-5o5p: Poliovirus type 3 (strain Saukett) stabilized virus-like particle... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5o5p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Poliovirus type 3 (strain Saukett) stabilized virus-like particle in complex with the pocket factor compound GPP3 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | VIRUS LIKE PARTICLE / Poliovirus / virus-like particle / vaccine / pocket factor | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont genome entry into host cell via pore formation in plasma membrane / viral capsid / host cell cytoplasm / symbiont-mediated suppression of host gene expression / virion attachment to host cell / structural molecule activity Similarity search - Function | |||||||||
| Biological species |  Human poliovirus 3 Human poliovirus 3 | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Bahar, M.W. / Kotecha, A. / Fry, E.E. / Stuart, D.I. | |||||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2017 Journal: Nat Commun / Year: 2017Title: Plant-made polio type 3 stabilized VLPs-a candidate synthetic polio vaccine. Authors: Johanna Marsian / Helen Fox / Mohammad W Bahar / Abhay Kotecha / Elizabeth E Fry / David I Stuart / Andrew J Macadam / David J Rowlands / George P Lomonossoff /  Abstract: Poliovirus (PV) is the causative agent of poliomyelitis, a crippling human disease known since antiquity. PV occurs in two distinct antigenic forms, D and C, of which only the D form elicits a robust ...Poliovirus (PV) is the causative agent of poliomyelitis, a crippling human disease known since antiquity. PV occurs in two distinct antigenic forms, D and C, of which only the D form elicits a robust neutralizing response. Developing a synthetically produced stabilized virus-like particle (sVLP)-based vaccine with D antigenicity, without the drawbacks of current vaccines, will be a major step towards the final eradication of poliovirus. Such a sVLP would retain the native antigenic conformation and the repetitive structure of the original virus particle, but lack infectious genomic material. In this study, we report the production of synthetically stabilized PV VLPs in plants. Mice carrying the gene for the human PV receptor are protected from wild-type PV when immunized with the plant-made PV sVLPs. Structural analysis of the stabilized mutant at 3.6 Å resolution by cryo-electron microscopy and single-particle reconstruction reveals a structure almost indistinguishable from wild-type PV3.Despite the success of current vaccination against poliomyelitis, safe, cheap and effective vaccines remain sought for continuing eradication effort. Here the authors use plants to express stabilized virus-like particles of type 3 poliovirus that can induce a protective immune response in mice transgenic for the human poliovirus receptor. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5o5p.cif.gz 5o5p.cif.gz | 149.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5o5p.ent.gz pdb5o5p.ent.gz | 113.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5o5p.json.gz 5o5p.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/o5/5o5p https://data.pdbj.org/pub/pdb/validation_reports/o5/5o5p ftp://data.pdbj.org/pub/pdb/validation_reports/o5/5o5p ftp://data.pdbj.org/pub/pdb/validation_reports/o5/5o5p | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3749MC  3747C  5o5bC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 | x 60
|
| 2 |
|
| 3 | x 5
|
| 4 | x 6
|
| 5 | 
|
- Components
Components
| #1: Protein | Mass: 33562.785 Da / Num. of mol.: 1 / Mutation: T105M, F132L Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host: Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host:  |
|---|---|
| #2: Protein | Mass: 30188.982 Da / Num. of mol.: 1 / Mutation: L18I, L215M, D241E Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host: Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host:  |
| #3: Protein | Mass: 26315.100 Da / Num. of mol.: 1 / Mutation: H19Y, L85F Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host: Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host:  |
| #4: Protein | Mass: 7452.113 Da / Num. of mol.: 1 / Mutation: T67A Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host: Human poliovirus 3 / Strain: Saukett / Plasmid: pEAQ-HT / Production host:  |
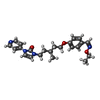
| #5: Chemical | ChemComp-9LW / |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human poliovirus 3 / Type: VIRUS Details: Poliovirus type 3 (Saukett strain) virus-like particle produced in plant expression system. Entity ID: #1-#4 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 5.8 MDa / Experimental value: NO |
| Source (natural) | Organism:  Human poliovirus 3 / Strain: Saukett Human poliovirus 3 / Strain: Saukett |
| Source (recombinant) | Organism:  |
| Details of virus | Empty: YES / Enveloped: NO / Isolate: SEROTYPE / Type: VIRUS-LIKE PARTICLE |
| Natural host | Organism: Human poliovirus 3 / Strain: Saukett |
| Virus shell | Name: Capsid / Diameter: 327 nm / Triangulation number (T number): 1 |
| Buffer solution | pH: 7 / Details: PBS pH 7.0 |
| Specimen | Conc.: 0.1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: Virus-like particles for polio type 3 (strain Saukett) in complex with pocket factor compound GPP3 were assessed by negative stain EM analysis, prior to cryo-em data collection. |
| Specimen support | Grid material: COPPER / Grid mesh size: 200 divisions/in. / Grid type: C-flat CF-2/1-2C |
| Vitrification | Instrument: FEI VITROBOT MARK I / Cryogen name: ETHANE / Humidity: 90 % / Chamber temperature: 293 K / Details: Blot for 4 seconds before plunging. |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI POLARA 300 / Details: Preliminary grid screening was performed. |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Calibrated magnification: 37037 X / Nominal defocus max: 2800 nm / Nominal defocus min: 800 nm / Cs: 2 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN Specimen holder model: GATAN 910 MULTI-SPECIMEN SINGLE TILT CRYO TRANSFER HOLDER |
| Image recording | Average exposure time: 0.2 sec. / Electron dose: 1.2 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 1018 |
| EM imaging optics | Energyfilter name: GIF Quantum / Energyfilter upper: 20 eV / Energyfilter lower: 0 eV |
| Image scans | Sampling size: 5 µm / Width: 4086 / Height: 4086 / Movie frames/image: 25 / Used frames/image: 2-25 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.12rc0_2787: / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Image processing | Details: The selected images were drift corrected using MotionCorr. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Details: CTF parameters were estimated using CTFFIND3 as part of RELION 1.3. Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 6639 Details: Automated particle picking (ETHAN) was followed by manual cleaning (EMAN2). | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: I (icosahedral) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 2060 / Algorithm: BACK PROJECTION / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL / Target criteria: Cross-correlation coefficient Details: phenix.real_space_refine was used to refine the atomic model in the cryo-em map. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj