+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 5jlh | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
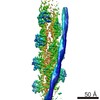
| タイトル | Cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution | |||||||||
 要素 要素 |
| |||||||||
 キーワード キーワード | CONTRACTILE PROTEIN / CONTRACTILE FILAMENT / MUSCLE / THIN FILAMENT / CYTOSKELETON / STRUCTURAL PROTEIN / HYDROLASE COMPLEX / F-actin / tropomyosin / filament / myosin / protein polymers / cryo EM | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報RHOBTB2 GTPase cycle / RHOU GTPase cycle / RHOV GTPase cycle / contractile vacuole / COPI-mediated anterograde transport / Platelet degranulation / sorocarp development / basal body patch / myosin II filament / macropinocytic cup ...RHOBTB2 GTPase cycle / RHOU GTPase cycle / RHOV GTPase cycle / contractile vacuole / COPI-mediated anterograde transport / Platelet degranulation / sorocarp development / basal body patch / myosin II filament / macropinocytic cup / Neutrophil degranulation / tight junction assembly / actin crosslink formation / profilin binding / actin filament-based movement / vocalization behavior / protein localization to bicellular tight junction / regulation of transepithelial transport / Formation of annular gap junctions / Formation of the dystrophin-glycoprotein complex (DGC) / morphogenesis of a polarized epithelium / actomyosin / structural constituent of postsynaptic actin cytoskeleton / Gap junction degradation / Cell-extracellular matrix interactions / myosin filament / dense body / regulation of stress fiber assembly / RHO GTPases activate CIT / RHO GTPases Activate ROCKs / actomyosin structure organization / Sema4D induced cell migration and growth-cone collapse / Adherens junctions interactions / myosin II complex / hyperosmotic response / Sensory processing of sound by outer hair cells of the cochlea / Interaction between L1 and Ankyrins / sarcomere organization / Sensory processing of sound by inner hair cells of the cochlea / regulation of focal adhesion assembly / EPHA-mediated growth cone collapse / cortical actin cytoskeleton / positive regulation of wound healing / apical junction complex / microfilament motor activity / maintenance of blood-brain barrier / filamentous actin / myofibril / NuA4 histone acetyltransferase complex / pseudopodium / cell leading edge / Recycling pathway of L1 / RHO GTPases activate PAKs / brush border / mitotic cytokinesis / actin filament bundle assembly / EPH-ephrin mediated repulsion of cells / RHO GTPases Activate WASPs and WAVEs / regulation of synaptic vesicle endocytosis / skeletal muscle contraction / phagocytosis / RHO GTPases activate IQGAPs / RHOBTB2 GTPase cycle / neuronal action potential / skeletal muscle tissue development / RHO GTPases activate PKNs / stress fiber / phagocytic vesicle / EPHB-mediated forward signaling / extracellular matrix / axonogenesis / calyx of Held / cellular response to starvation / Translocation of SLC2A4 (GLUT4) to the plasma membrane / cell projection / FCGR3A-mediated phagocytosis / actin filament / mitochondrion organization / cell motility / sensory perception of sound / RHO GTPases Activate Formins / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / Regulation of actin dynamics for phagocytic cup formation / cellular response to type II interferon / structural constituent of cytoskeleton / 加水分解酵素; 酸無水物に作用; 酸無水物に作用・細胞または細胞小器官の運動に関与 / VEGFA-VEGFR2 Pathway / platelet aggregation / Schaffer collateral - CA1 synapse / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / actin filament binding / cell-cell junction / Signaling by BRAF and RAF1 fusions / regulation of cell shape / actin cytoskeleton / Clathrin-mediated endocytosis 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 電子顕微鏡法 / 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.9 Å | |||||||||
 データ登録者 データ登録者 | von der Ecken, J. / Heissler, S.M. / Pathan-Chhatbar, S. / Manstein, D.J. / Raunser, S. | |||||||||
| 資金援助 |  ドイツ, 2件 ドイツ, 2件
| |||||||||
 引用 引用 |  ジャーナル: Nature / 年: 2016 ジャーナル: Nature / 年: 2016タイトル: Cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution. 要旨: The interaction of myosin with actin filaments is the central feature of muscle contraction and cargo movement along actin filaments of the cytoskeleton. The energy for these movements is generated ...The interaction of myosin with actin filaments is the central feature of muscle contraction and cargo movement along actin filaments of the cytoskeleton. The energy for these movements is generated during a complex mechanochemical reaction cycle. Crystal structures of myosin in different states have provided important structural insights into the myosin motor cycle when myosin is detached from F-actin. The difficulty of obtaining diffracting crystals, however, has prevented structure determination by crystallography of actomyosin complexes. Thus, although structural models exist of F-actin in complex with various myosins, a high-resolution structure of the F-actin–myosin complex is missing. Here, using electron cryomicroscopy, we present the structure of a human rigor actomyosin complex at an average resolution of 3.9 Å. The structure reveals details of the actomyosin interface, which is mainly stabilized by hydrophobic interactions. The negatively charged amino (N) terminus of actin interacts with a conserved basic motif in loop 2 of myosin, promoting cleft closure in myosin. Surprisingly, the overall structure of myosin is similar to rigor-like myosin structures in the absence of F-actin, indicating that F-actin binding induces only minimal conformational changes in myosin. A comparison with pre-powerstroke and intermediate (Pi-release) states of myosin allows us to discuss the general mechanism of myosin binding to F-actin. Our results serve as a strong foundation for the molecular understanding of cytoskeletal diseases, such as autosomal dominant hearing loss and diseases affecting skeletal and cardiac muscles, in particular nemaline myopathy and hypertrophic cardiomyopathy. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  5jlh.cif.gz 5jlh.cif.gz | 735.5 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb5jlh.ent.gz pdb5jlh.ent.gz | 588.6 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  5jlh.json.gz 5jlh.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  5jlh_validation.pdf.gz 5jlh_validation.pdf.gz | 1.1 MB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  5jlh_full_validation.pdf.gz 5jlh_full_validation.pdf.gz | 1.2 MB | 表示 | |
| XML形式データ |  5jlh_validation.xml.gz 5jlh_validation.xml.gz | 101.4 KB | 表示 | |
| CIF形式データ |  5jlh_validation.cif.gz 5jlh_validation.cif.gz | 155.4 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/jl/5jlh https://data.pdbj.org/pub/pdb/validation_reports/jl/5jlh ftp://data.pdbj.org/pub/pdb/validation_reports/jl/5jlh ftp://data.pdbj.org/pub/pdb/validation_reports/jl/5jlh | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 非結晶学的対称性 (NCS) | NCSドメイン:
NCSドメイン領域:
NCSアンサンブル:
NCS oper:
|
- 要素
要素
| #1: タンパク質 | 分子量: 41707.570 Da / 分子数: 5 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: ACTG1, ACTG Homo sapiens (ヒト) / 遺伝子: ACTG1, ACTG発現宿主:  参照: UniProt: P63261 #2: タンパク質 | 分子量: 117570.633 Da / 分子数: 2 / 由来タイプ: 組換発現 詳細: NON MUSCLE MYOSIN 2C HEAVY CHAIN, MOTOR DOMAIN RESIDUES 1-799 LINKED TO ALPHA-ACTININ 3, REPEATS 1 AND 2 RESIDUES 800-1039 FRAGMENT: UNP Q7Z406-1 RESIDUES 1-799, UNP P05095 RESIDUES 265-502 由来: (組換発現)  Homo sapiens (ヒト), (組換発現) Homo sapiens (ヒト), (組換発現)  遺伝子: MYH14, KIAA2034, FP17425, abpA, actnA, DDB_G0268632 発現宿主:  参照: UniProt: Q7Z406, UniProt: P05095 #3: タンパク質 | 分子量: 11507.176 Da / 分子数: 4 / 由来タイプ: 組換発現 詳細: Human TROPOMYSIN WAS USED (UNP P06753-2,TPM3_HUMAN, RESIDUES 62-196). DUE TO THE LIMITED RESOLUTION OF THE CRYO-EM DENSITY IN THE REGION OF TROPOMYOSIN, TROPOMYOSIN HAS BEEN REPRESENTED AS POLY(UNK). 由来: (組換発現)  Homo sapiens (ヒト) / 発現宿主: Homo sapiens (ヒト) / 発現宿主:  #4: 化合物 | ChemComp-ADP / #5: 化合物 | ChemComp-MG / 配列の詳細 | Human TROPOMYSIN WAS USED (UNP P06753-2,TPM3_HUMAN, RESIDUES 62-196) DUE TO THE LIMITED RESOLUTION ...Human TROPOMYSIN | |
|---|
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: FILAMENT / 3次元再構成法: 単粒子再構成法 |
- 試料調製
試料調製
| 構成要素 |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 分子量 | 値: 0.44 MDa / 実験値: NO | ||||||||||||||||||||||||||||
| 由来(天然) |
| ||||||||||||||||||||||||||||
| 由来(組換発現) |
| ||||||||||||||||||||||||||||
| 緩衝液 | pH: 7.5 詳細: 5 mM Tris-HCl pH 7.5, 1 mM DTT, 100 mM KCl, and 2 mM MgCl2 | ||||||||||||||||||||||||||||
| 緩衝液成分 |
| ||||||||||||||||||||||||||||
| 試料 | 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES | ||||||||||||||||||||||||||||
| 試料支持 | グリッドの材料: COPPER / グリッドのサイズ: 300 divisions/in. / グリッドのタイプ: C-flat-2/1 | ||||||||||||||||||||||||||||
| EM embedding | Material: I | ||||||||||||||||||||||||||||
| 急速凍結 | 装置: GATAN CRYOPLUNGE 3 / 凍結剤: ETHANE / 湿度: 90 % 詳細: Sample (2 uL of F-actin-tropomyosin solution) was applied to a glow-discharged holey carbon grid, incubated for 20 s and manually blotted from the backside for less than a second with filter ...詳細: Sample (2 uL of F-actin-tropomyosin solution) was applied to a glow-discharged holey carbon grid, incubated for 20 s and manually blotted from the backside for less than a second with filter paper. Afterwards 1.5 uL of myosin solution (3 uM without nucleotide) were added directly on the grid, incubated for 10 s and then manually blotted for 5 s from the backside with filter paper. |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
|---|---|
| 顕微鏡 | モデル: FEI TITAN KRIOS / 詳細: Cs corrected microscope |
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: OTHER FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: OTHER |
| 電子レンズ | モード: BRIGHT FIELD / 倍率(公称値): 59000 X / Calibrated defocus min: 700 nm / 最大 デフォーカス(補正後): 2800 nm |
| 撮影 | 平均露光時間: 0.475 sec. / 電子線照射量: 16 e/Å2 / 検出モード: COUNTING フィルム・検出器のモデル: FEI FALCON II (4k x 4k) 実像数: 6300 |
| 画像スキャン | 動画フレーム数/画像: 8 / 利用したフレーム数/画像: 2-8 |
- 解析
解析
| ソフトウェア | 名称: REFMAC / バージョン: 5.8.0088 / 分類: 精密化 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMソフトウェア |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF補正 | タイプ: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| らせん対称 | 回転角度/サブユニット: 166.9 ° / 軸方向距離/サブユニット: 27.5 Å / らせん対称軸の対称性: C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 粒子像の選択 | 選択した粒子像数: 138000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3次元再構成 | 解像度: 3.9 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 粒子像の数: 118000 詳細: THE TROPOMYOSIN MAP FILTERED TO 7.0 ANGSTROM WAS MERGED WITH THE FINAL F-ACTIN-MYOSIN MAP (3.9 ANGSTROM) TO OBTAIN A MAP OF THE ENTIRE F-ACTIN-MYOSIN-TROPOMYOSIN COMPLEX. 対称性のタイプ: HELICAL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子モデル構築 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子モデル構築 | Source name: PDB / タイプ: experimental model
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化 | 解像度: 3.9→211.2 Å / Cor.coef. Fo:Fc: 0.907 / SU B: 27.204 / SU ML: 0.36 / ESU R: 0.558 立体化学のターゲット値: MAXIMUM LIKELIHOOD WITH PHASES 詳細: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS MERGED WITH THE FINAL F-ACTIN-MUYOSIN MAP (3.9 ANGSTROM) TO OBTAIN MAP OF THE ENTIRE F-ACTIN TROPOMYOSIN COMPLEX.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 溶媒の処理 | イオンプローブ半径: 0.8 Å / 減衰半径: 0.8 Å / VDWプローブ半径: 1.2 Å / 溶媒モデル: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子変位パラメータ | Biso mean: 180.474 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: 1 / 合計: 26477 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 |
|
 ムービー
ムービー コントローラー
コントローラー













 PDBj
PDBj




























