| Entry | Database: PDB / ID: 5hrq
|
|---|
| Title | Insulin with proline analog HzP at position B28 in the R6 state |
|---|
 Components Components | - Insulin A-Chain
- Insulin B-Chain
|
|---|
 Keywords Keywords | HORMONE / Insulin / non-canonical amino acid / hydroxyproline / non-natural amino acid / unnatural amino acid |
|---|
| Function / homology |  Function and homology information Function and homology information
negative regulation of glycogen catabolic process / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / negative regulation of feeding behavior / Signaling by Insulin receptor / IRS activation / Insulin processing / regulation of protein secretion / positive regulation of peptide hormone secretion / positive regulation of respiratory burst ...negative regulation of glycogen catabolic process / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / negative regulation of feeding behavior / Signaling by Insulin receptor / IRS activation / Insulin processing / regulation of protein secretion / positive regulation of peptide hormone secretion / positive regulation of respiratory burst / Regulation of gene expression in beta cells / negative regulation of acute inflammatory response / alpha-beta T cell activation / Synthesis, secretion, and deacylation of Ghrelin / positive regulation of dendritic spine maintenance / negative regulation of protein secretion / negative regulation of gluconeogenesis / positive regulation of glycogen biosynthetic process / fatty acid homeostasis / Signal attenuation / positive regulation of insulin receptor signaling pathway / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / negative regulation of respiratory burst involved in inflammatory response / negative regulation of lipid catabolic process / positive regulation of lipid biosynthetic process / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / regulation of protein localization to plasma membrane / transport vesicle / nitric oxide-cGMP-mediated signaling / positive regulation of nitric-oxide synthase activity / COPI-mediated anterograde transport / Insulin receptor recycling / negative regulation of reactive oxygen species biosynthetic process / positive regulation of brown fat cell differentiation / insulin-like growth factor receptor binding / NPAS4 regulates expression of target genes / neuron projection maintenance / endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of mitotic nuclear division / Insulin receptor signalling cascade / positive regulation of glycolytic process / positive regulation of cytokine production / endosome lumen / acute-phase response / positive regulation of long-term synaptic potentiation / positive regulation of D-glucose import across plasma membrane / positive regulation of protein secretion / insulin receptor binding / positive regulation of cell differentiation / Regulation of insulin secretion / wound healing / positive regulation of neuron projection development / hormone activity / negative regulation of protein catabolic process / regulation of synaptic plasticity / positive regulation of protein localization to nucleus / Golgi lumen / vasodilation / cognition / glucose metabolic process / insulin receptor signaling pathway / cell-cell signaling / glucose homeostasis / regulation of protein localization / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / positive regulation of cell growth / protease binding / secretory granule lumen / positive regulation of canonical NF-kappaB signal transduction / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of MAPK cascade / positive regulation of cell migration / G protein-coupled receptor signaling pathway / endoplasmic reticulum lumen / Amyloid fiber formation / receptor ligand activity / Golgi membrane / negative regulation of gene expression / positive regulation of cell population proliferation / positive regulation of gene expression / regulation of DNA-templated transcription / extracellular space / extracellular region / identical protein bindingSimilarity search - Function Insulin / Insulin family / Insulin-like / Insulin/IGF/Relaxin family / Insulin / insulin-like growth factor / relaxin family. / Insulin, conserved site / Insulin family signature. / Insulin-like superfamilySimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.28 Å molecular replacement / Resolution: 1.28 Å |
|---|
 Authors Authors | Lieblich, S.A. / Fang, K.Y. / Cahn, J.K.B. / Tirrell, D.A. |
|---|
| Funding support |  Denmark, 1items Denmark, 1items | Organization | Grant number | Country |
|---|
| Novo Nordisk Foundation | |  Denmark Denmark |
|
|---|
 Citation Citation |  Journal: J. Am. Chem. Soc. / Year: 2017 Journal: J. Am. Chem. Soc. / Year: 2017
Title: 4S-Hydroxylation of Insulin at ProB28 Accelerates Hexamer Dissociation and Delays Fibrillation.
Authors: Lieblich, S.A. / Fang, K.Y. / Cahn, J.K.B. / Rawson, J. / LeBon, J. / Ku, H.T. / Tirrell, D.A. |
|---|
| History | | Deposition | Jan 24, 2016 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Jan 25, 2017 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Jul 12, 2017 | Group: Database references / Category: citation / citation_author
Item: _citation.country / _citation.journal_abbrev ..._citation.country / _citation.journal_abbrev / _citation.journal_id_ASTM / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year |
|---|
| Revision 1.2 | Jan 15, 2020 | Group: Author supporting evidence / Category: pdbx_audit_support / Item: _pdbx_audit_support.funding_organization |
|---|
| Revision 1.3 | Apr 2, 2025 | Group: Data collection / Database references ...Data collection / Database references / Derived calculations / Structure summary
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature / struct_conn / struct_conn_type
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_conn_type.id |
|---|
|
|---|
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.28 Å
molecular replacement / Resolution: 1.28 Å  Authors
Authors Denmark, 1items
Denmark, 1items  Citation
Citation Journal: J. Am. Chem. Soc. / Year: 2017
Journal: J. Am. Chem. Soc. / Year: 2017 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5hrq.cif.gz
5hrq.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5hrq.ent.gz
pdb5hrq.ent.gz PDB format
PDB format 5hrq.json.gz
5hrq.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/hr/5hrq
https://data.pdbj.org/pub/pdb/validation_reports/hr/5hrq ftp://data.pdbj.org/pub/pdb/validation_reports/hr/5hrq
ftp://data.pdbj.org/pub/pdb/validation_reports/hr/5hrq Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: INS / Plasmid: pQE80L / Production host:
Homo sapiens (human) / Gene: INS / Plasmid: pQE80L / Production host: 
 Homo sapiens (human) / Gene: INS / Plasmid: pQE80L / Production host:
Homo sapiens (human) / Gene: INS / Plasmid: pQE80L / Production host: 
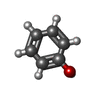






 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SSRL
SSRL  / Beamline: BL12-2 / Wavelength: 0.9795 Å
/ Beamline: BL12-2 / Wavelength: 0.9795 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT / Resolution: 1.28→56.7 Å / Cor.coef. Fo:Fc: 0.977 / Cor.coef. Fo:Fc free: 0.968 / SU B: 1.348 / SU ML: 0.026 / Cross valid method: THROUGHOUT / ESU R: 0.04 / ESU R Free: 0.042 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
MOLECULAR REPLACEMENT / Resolution: 1.28→56.7 Å / Cor.coef. Fo:Fc: 0.977 / Cor.coef. Fo:Fc free: 0.968 / SU B: 1.348 / SU ML: 0.026 / Cross valid method: THROUGHOUT / ESU R: 0.04 / ESU R Free: 0.042 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS Movie
Movie Controller
Controller















 PDBj
PDBj



















