[English] 日本語
 Yorodumi
Yorodumi- PDB-5hj1: Crystal structure of PDZ domain of pullulanase C protein of type ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5hj1 | ||||||
|---|---|---|---|---|---|---|---|
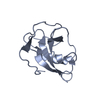
| Title | Crystal structure of PDZ domain of pullulanase C protein of type II secretion system from Klebsiella pneumoniae in complex with fatty acid | ||||||
 Components Components | Pullulanase C protein | ||||||
 Keywords Keywords | HYDROLASE / GspC protein / Structural Genomics / CSGID / Center for Structural Genomics of Infectious Diseases | ||||||
| Function / homology | PDZ domain / Pdz3 Domain / Roll / Mainly Beta / VACCENIC ACID / :  Function and homology information Function and homology information | ||||||
| Biological species |  Klebsiella pneumoniae subsp. pneumoniae NTUH-K2044 (bacteria) Klebsiella pneumoniae subsp. pneumoniae NTUH-K2044 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 1.5 Å SAD / Resolution: 1.5 Å | ||||||
 Authors Authors | Filippova, E.V. / Minasov, G. / Shuvalova, L. / Kiryukhina, O. / Dubrovska, I. / Grimshaw, S. / Kwon, K. / Anderson, W.F. / Center for Structural Genomics of Infectious Diseases (CSGID) | ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Crystal structure of PDZ domain of pullulanase C protein of type II secretion system from Klebsiella pneumoniae in complex with fatty acid Authors: Filippova, E.V. / Minasov, G. / Shuvalova, L. / Kiryukhina, O. / Dubrovska, I. / Grimshaw, S. / Kwon, K. / Anderson, W.F. / Center for Structural Genomics of Infectious Diseases (CSGID) | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5hj1.cif.gz 5hj1.cif.gz | 58.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5hj1.ent.gz pdb5hj1.ent.gz | 41.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5hj1.json.gz 5hj1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hj/5hj1 https://data.pdbj.org/pub/pdb/validation_reports/hj/5hj1 ftp://data.pdbj.org/pub/pdb/validation_reports/hj/5hj1 ftp://data.pdbj.org/pub/pdb/validation_reports/hj/5hj1 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 11429.631 Da / Num. of mol.: 1 / Fragment: PDZ domain (UNP residues 181-280) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Klebsiella pneumoniae subsp. pneumoniae NTUH-K2044 (bacteria) Klebsiella pneumoniae subsp. pneumoniae NTUH-K2044 (bacteria)Gene: YP_002917874 / Plasmid: pMCSG53 / Production host:  |
|---|---|
| #2: Chemical | ChemComp-VCA / |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
| Nonpolymer details | The ligand was identified based on the electron density maps and was not originally present in ...The ligand was identified based on the electron density maps and was not originally present in protein solution or crystallization conditions. Based on the results described by R. Mejia, et al., 1999 authors concluded that vaccenic acid is likely to bind to a protein. |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 47.04 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, sitting drop / Details: 4 M Formate |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-F / Wavelength: 0.97872 Å / Beamline: 21-ID-F / Wavelength: 0.97872 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Dec 14, 2015 |
| Radiation | Monochromator: C(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97872 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→50 Å / Num. obs: 17852 / % possible obs: 99.6 % / Redundancy: 27.7 % / Rmerge(I) obs: 0.06 / Net I/σ(I): 87.8 |
| Reflection shell | Resolution: 1.5→1.53 Å / Redundancy: 28.3 % / Rmerge(I) obs: 0.57 / Mean I/σ(I) obs: 6.9 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 1.5→42.42 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.95 / SU B: 2.181 / SU ML: 0.041 / Cross valid method: THROUGHOUT / ESU R: 0.077 / ESU R Free: 0.079 / Stereochemistry target values: MAXIMUM LIKELIHOOD SAD / Resolution: 1.5→42.42 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.95 / SU B: 2.181 / SU ML: 0.041 / Cross valid method: THROUGHOUT / ESU R: 0.077 / ESU R Free: 0.079 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. The ligand was identified based on the electron density maps and was not originally present in protein solution or crystallization ...Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. The ligand was identified based on the electron density maps and was not originally present in protein solution or crystallization conditions. Based on the results described by R. Mejia, et al., 1999 authors concluded that vaccenic acid is likely to bind to a protein.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 32.17 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→42.42 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj