Entry Database : PDB / ID : 5hiyTitle Crystal structure of PEDV NSP9 Mutant-C59A Non-structural protein 9 Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Method / / / Resolution : 3 Å Authors Deng, F. / Peng, G. Journal : J.Virol. / Year : 2018Title : Dimerization of Coronavirus nsp9 with Diverse Modes Enhances Its Nucleic Acid Binding Affinity.Authors : Zeng, Z. / Deng, F. / Shi, K. / Ye, G. / Wang, G. / Fang, L. / Xiao, S. / Fu, Z. / Peng, G. History Deposition Jan 12, 2016 Deposition site / Processing site Revision 1.0 Jan 25, 2017 Provider / Type Revision 1.1 Jul 3, 2019 Group / Database references / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_ASTM / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year Revision 1.2 Mar 20, 2024 Group / Database references / Category / chem_comp_bond / database_2Item / _database_2.pdbx_database_accession
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Porcine epidemic diarrhea virus CV777
Porcine epidemic diarrhea virus CV777 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3 Å
MOLECULAR REPLACEMENT / Resolution: 3 Å  Authors
Authors Citation
Citation Journal: J.Virol. / Year: 2018
Journal: J.Virol. / Year: 2018 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5hiy.cif.gz
5hiy.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5hiy.ent.gz
pdb5hiy.ent.gz PDB format
PDB format 5hiy.json.gz
5hiy.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/hi/5hiy
https://data.pdbj.org/pub/pdb/validation_reports/hi/5hiy ftp://data.pdbj.org/pub/pdb/validation_reports/hi/5hiy
ftp://data.pdbj.org/pub/pdb/validation_reports/hi/5hiy Links
Links Assembly
Assembly


 Components
Components Porcine epidemic diarrhea virus CV777 / Strain: CV777 / Gene: rep, 1a-1b
Porcine epidemic diarrhea virus CV777 / Strain: CV777 / Gene: rep, 1a-1b
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SSRF
SSRF  / Beamline: BL17U / Wavelength: 0.97917 Å
/ Beamline: BL17U / Wavelength: 0.97917 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 3→50 Å / Cross valid method: FREE R-VALUE
MOLECULAR REPLACEMENT / Resolution: 3→50 Å / Cross valid method: FREE R-VALUE Movie
Movie Controller
Controller















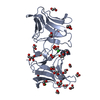
 PDBj
PDBj





