[English] 日本語
 Yorodumi
Yorodumi- PDB-5gpg: Co-crystal structure of the FK506 binding domain of human FKBP25,... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5gpg | ||||||
|---|---|---|---|---|---|---|---|
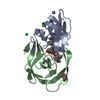
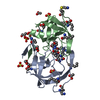
| Title | Co-crystal structure of the FK506 binding domain of human FKBP25, Rapamycin and the FRB domain of human mTOR | ||||||
 Components Components |
| ||||||
 Keywords Keywords | ISOMERASE/TRANSFERASE / complex / kinase / ISOMERASE-TRANSFERASE complex | ||||||
| Function / homology |  Function and homology information Function and homology informationRNA polymerase III type 2 promoter sequence-specific DNA binding / RNA polymerase III type 1 promoter sequence-specific DNA binding / positive regulation of cytoplasmic translational initiation / regulation of locomotor rhythm / T-helper 1 cell lineage commitment / positive regulation of pentose-phosphate shunt / positive regulation of wound healing, spreading of epidermal cells / TORC2 complex / regulation of membrane permeability / TORC2 signaling ...RNA polymerase III type 2 promoter sequence-specific DNA binding / RNA polymerase III type 1 promoter sequence-specific DNA binding / positive regulation of cytoplasmic translational initiation / regulation of locomotor rhythm / T-helper 1 cell lineage commitment / positive regulation of pentose-phosphate shunt / positive regulation of wound healing, spreading of epidermal cells / TORC2 complex / regulation of membrane permeability / TORC2 signaling / cellular response to leucine starvation / TFIIIC-class transcription factor complex binding / heart valve morphogenesis / negative regulation of lysosome organization / TORC1 complex / voluntary musculoskeletal movement / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / calcineurin-NFAT signaling cascade / RNA polymerase III type 3 promoter sequence-specific DNA binding / positive regulation of keratinocyte migration / regulation of osteoclast differentiation / MTOR signalling / regulation of lysosome organization / energy reserve metabolic process / cellular response to L-leucine / regulation of autophagosome assembly / Energy dependent regulation of mTOR by LKB1-AMPK / cellular response to nutrient / Amino acids regulate mTORC1 / cellular response to methionine / serine/threonine protein kinase complex / ruffle organization / negative regulation of cell size / positive regulation of ubiquitin-dependent protein catabolic process / cellular response to osmotic stress / anoikis / inositol hexakisphosphate binding / negative regulation of protein localization to nucleus / cardiac muscle cell development / negative regulation of calcineurin-NFAT signaling cascade / regulation of myelination / positive regulation of transcription by RNA polymerase III / negative regulation of macroautophagy / TORC1 signaling / positive regulation of myotube differentiation / Macroautophagy / FK506 binding / regulation of cell size / Constitutive Signaling by AKT1 E17K in Cancer / positive regulation of actin filament polymerization / germ cell development / behavioral response to pain / oligodendrocyte differentiation / positive regulation of oligodendrocyte differentiation / TOR signaling / positive regulation of translational initiation / mTORC1-mediated signalling / CD28 dependent PI3K/Akt signaling / HSF1-dependent transactivation / regulation of macroautophagy / 'de novo' pyrimidine nucleobase biosynthetic process / response to amino acid / positive regulation of epithelial to mesenchymal transition / positive regulation of lipid biosynthetic process / heart morphogenesis / vascular endothelial cell response to laminar fluid shear stress / regulation of cellular response to heat / neuronal action potential / positive regulation of lamellipodium assembly / cardiac muscle contraction / positive regulation of stress fiber assembly / T cell costimulation / phagocytic vesicle / cellular response to nutrient levels / cytoskeleton organization / endomembrane system / negative regulation of insulin receptor signaling pathway / negative regulation of autophagy / cellular response to amino acid starvation / cellular response to starvation / positive regulation of glycolytic process / Regulation of PTEN gene transcription / regulation of signal transduction by p53 class mediator / positive regulation of translation / VEGFR2 mediated vascular permeability / TP53 Regulates Metabolic Genes / post-embryonic development / regulation of actin cytoskeleton organization / peptidylprolyl isomerase / non-specific protein-tyrosine kinase / macroautophagy / peptidyl-prolyl cis-trans isomerase activity / regulation of cell growth / cellular response to amino acid stimulus / phosphoprotein binding / response to nutrient levels / regulation of circadian rhythm / PML body / protein destabilization / multicellular organism growth Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.67 Å MOLECULAR REPLACEMENT / Resolution: 1.67 Å | ||||||
 Authors Authors | Lee, H.B. / Lee, S.Y. / Rhee, H.W. / Lee, C.W. | ||||||
 Citation Citation |  Journal: Acs Cent.Sci. / Year: 2016 Journal: Acs Cent.Sci. / Year: 2016Title: Proximity-Directed Labeling Reveals a New Rapamycin-Induced Heterodimer of FKBP25 and FRB in Live Cells Authors: Lee, S.Y. / Lee, H. / Lee, H.K. / Lee, S.W. / Ha, S.C. / Kwon, T. / Seo, J.K. / Lee, C. / Rhee, H.W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5gpg.cif.gz 5gpg.cif.gz | 66 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5gpg.ent.gz pdb5gpg.ent.gz | 45.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5gpg.json.gz 5gpg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gp/5gpg https://data.pdbj.org/pub/pdb/validation_reports/gp/5gpg ftp://data.pdbj.org/pub/pdb/validation_reports/gp/5gpg ftp://data.pdbj.org/pub/pdb/validation_reports/gp/5gpg | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 13047.042 Da / Num. of mol.: 1 / Fragment: UNP residues 109-224 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: FKBP3, FKBP25 / Production host: Homo sapiens (human) / Gene: FKBP3, FKBP25 / Production host:  |
|---|---|
| #2: Protein | Mass: 11352.787 Da / Num. of mol.: 1 / Fragment: UNP residues 2021-2112 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MTOR, FRAP, FRAP1, FRAP2, RAFT1, RAPT1 / Production host: Homo sapiens (human) / Gene: MTOR, FRAP, FRAP1, FRAP2, RAFT1, RAPT1 / Production host:  References: UniProt: P42345, non-specific serine/threonine protein kinase |
| #3: Chemical | ChemComp-RAP / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.47 Å3/Da / Density % sol: 50.2 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 30% polyethyleneglycol 8000, 100mM sodium cacodylate, 200mM sodium acetate, 10mM manganese chloride |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: PAL/PLS SYNCHROTRON / Site: PAL/PLS  / Beamline: 7A (6B, 6C1) / Wavelength: 1 Å / Beamline: 7A (6B, 6C1) / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 270 / Detector: CCD / Date: Feb 17, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.669→50 Å / Num. obs: 27591 / % possible obs: 99.3 % / Redundancy: 5.2 % / Rmerge(I) obs: 0.081 / Net I/σ(I): 27.1 |
| Reflection shell | Resolution: 1.67→1.7 Å / Rmerge(I) obs: 0.512 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1PBK, 1FAP Resolution: 1.67→27.73 Å / SU ML: 0.16 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 20.7
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.67→27.73 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj