[English] 日本語
 Yorodumi
Yorodumi- PDB-5ezv: X-ray crystal structure of AMP-activated protein kinase alpha-2/a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ezv | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-ray crystal structure of AMP-activated protein kinase alpha-2/alpha-1 RIM chimaera (alpha-2(1-347)/alpha-1(349-401)/alpha-2(397-end) beta-1 gamma-1) co-crystallized with C2 (5-(5-hydroxyl-isoxazol-3-yl)-furan-2-phosphonic acid) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE / serine/threonine kinase / allosteric activation / nucleotide-binding | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of glucosylceramide biosynthetic process / nail development / [hydroxymethylglutaryl-CoA reductase (NADPH)] kinase / [hydroxymethylglutaryl-CoA reductase (NADPH)] kinase activity / regulation of stress granule assembly / positive regulation of mitochondrial transcription / AMPK inhibits chREBP transcriptional activation activity / histone H2BS36 kinase activity / cold acclimation / cAMP-dependent protein kinase regulator activity ...negative regulation of glucosylceramide biosynthetic process / nail development / [hydroxymethylglutaryl-CoA reductase (NADPH)] kinase / [hydroxymethylglutaryl-CoA reductase (NADPH)] kinase activity / regulation of stress granule assembly / positive regulation of mitochondrial transcription / AMPK inhibits chREBP transcriptional activation activity / histone H2BS36 kinase activity / cold acclimation / cAMP-dependent protein kinase regulator activity / AMP-activated protein kinase activity / lipid droplet disassembly / Lipophagy / regulation of carbon utilization / positive regulation of skeletal muscle tissue development / CAMKK-AMPK signaling cascade / import into nucleus / regulation of vesicle-mediated transport / nucleotide-activated protein kinase complex / negative regulation of hepatocyte apoptotic process / Energy dependent regulation of mTOR by LKB1-AMPK / Carnitine shuttle / positive regulation of T cell mediated immune response to tumor cell / tau-protein kinase / protein kinase regulator activity / negative regulation of TOR signaling / Activation of PPARGC1A (PGC-1alpha) by phosphorylation / Nuclear events mediated by NFE2L2 / response to caffeine / regulation of glycolytic process / : / tau-protein kinase activity / cAMP-dependent protein kinase activity / protein localization to lipid droplet / negative regulation of tubulin deacetylation / AMP binding / cellular response to stress / Macroautophagy / cholesterol biosynthetic process / lipid biosynthetic process / response to muscle activity / fatty acid oxidation / motor behavior / positive regulation of protein kinase activity / cellular response to ethanol / positive regulation of macroautophagy / regulation of macroautophagy / fatty acid homeostasis / negative regulation of lipid catabolic process / cellular response to nutrient levels / response to UV / cellular response to glucose starvation / energy homeostasis / Activation of AMPK downstream of NMDARs / positive regulation of protein localization / positive regulation of adipose tissue development / negative regulation of TORC1 signaling / positive regulation of autophagy / protein serine/threonine/tyrosine kinase activity / positive regulation of gluconeogenesis / negative regulation of insulin receptor signaling pathway / cellular response to calcium ion / regulation of microtubule cytoskeleton organization / positive regulation of glycolytic process / response to activity / response to gamma radiation / TP53 Regulates Metabolic Genes / Translocation of SLC2A4 (GLUT4) to the plasma membrane / cellular response to glucose stimulus / positive regulation of cholesterol biosynthetic process / neuron cellular homeostasis / ADP binding / regulation of circadian rhythm / positive regulation of T cell activation / autophagy / response to estrogen / cellular response to xenobiotic stimulus / tau protein binding / glucose metabolic process / cellular response to hydrogen peroxide / Wnt signaling pathway / cytoplasmic stress granule / fatty acid biosynthetic process / rhythmic process / cellular response to prostaglandin E stimulus / glucose homeostasis / positive regulation of cold-induced thermogenesis / cellular response to oxidative stress / spermatogenesis / cellular response to hypoxia / Regulation of TP53 Activity through Phosphorylation / response to hypoxia / protein phosphorylation / protein kinase activity / non-specific serine/threonine protein kinase / regulation of cell cycle / nuclear speck / cilium / ciliary basal body / apical plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.99 Å MOLECULAR REPLACEMENT / Resolution: 2.99 Å | ||||||
 Authors Authors | Langendorf, C.G. / Kemp, B.E. | ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2016 Journal: Nat Commun / Year: 2016Title: Structural basis of allosteric and synergistic activation of AMPK by furan-2-phosphonic derivative C2 binding. Authors: Langendorf, C.G. / Ngoei, K.R. / Scott, J.W. / Ling, N.X. / Issa, S.M. / Gorman, M.A. / Parker, M.W. / Sakamoto, K. / Oakhill, J.S. / Kemp, B.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ezv.cif.gz 5ezv.cif.gz | 736.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ezv.ent.gz pdb5ezv.ent.gz | 603.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ezv.json.gz 5ezv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ez/5ezv https://data.pdbj.org/pub/pdb/validation_reports/ez/5ezv ftp://data.pdbj.org/pub/pdb/validation_reports/ez/5ezv ftp://data.pdbj.org/pub/pdb/validation_reports/ez/5ezv | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4zhxSC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AC
| #1: Protein | Mass: 63902.699 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PRKAA2, AMPK, AMPK2, PRKAA1, AMPK1 / Plasmid: pETDuet-1 / Production host: Homo sapiens (human) / Gene: PRKAA2, AMPK, AMPK2, PRKAA1, AMPK1 / Plasmid: pETDuet-1 / Production host:  References: UniProt: P54646, UniProt: Q13131, non-specific serine/threonine protein kinase, EC: 2.7.11.27, [hydroxymethylglutaryl-CoA reductase (NADPH)] kinase |
|---|
-5'-AMP-activated protein kinase subunit ... , 2 types, 4 molecules BDEF
| #2: Protein | Mass: 30504.299 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PRKAB1, AMPK / Plasmid: pCOLA / Production host: Homo sapiens (human) / Gene: PRKAB1, AMPK / Plasmid: pCOLA / Production host:  #3: Protein | Mass: 38225.914 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PRKAG1 / Plasmid: pETDuet-1 / Production host: Homo sapiens (human) / Gene: PRKAG1 / Plasmid: pETDuet-1 / Production host:  |
|---|
-Non-polymers , 4 types, 92 molecules 
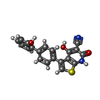




| #4: Chemical | | #5: Chemical | #6: Chemical | ChemComp-C2Z / #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.7 Å3/Da / Density % sol: 54.38 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 6.2 Details: 8 % PEG 3350, 0.1 M MgCl2, 1.0 % glucose, 0.001 % cocamidopropyl betaine, 0.1 M imidazole PH range: 6.2 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Australian Synchrotron Australian Synchrotron  / Beamline: MX2 / Wavelength: 0.9537 Å / Beamline: MX2 / Wavelength: 0.9537 Å | |||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Feb 6, 2015 / Details: mirrors | |||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9537 Å / Relative weight: 1 | |||||||||||||||||||||||||||
| Reflection | Resolution: 2.99→49.3 Å / Num. all: 182062 / Num. obs: 56584 / % possible obs: 99.4 % / Redundancy: 3.2 % / Biso Wilson estimate: 87.43 Å2 / CC1/2: 0.994 / Rmerge(I) obs: 0.081 / Rpim(I) all: 0.053 / Net I/σ(I): 10.1 / Num. measured all: 182062 / Scaling rejects: 24 | |||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4ZHX Resolution: 2.99→49.3 Å / Cor.coef. Fo:Fc: 0.9073 / Cor.coef. Fo:Fc free: 0.8933 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 4.496 / SU Rfree Blow DPI: 0.359
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 175.46 Å2 / Biso mean: 68.78 Å2 / Biso min: 20 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.48 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.99→49.3 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.99→3.07 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 119.3968 Å / Origin y: -35.0872 Å / Origin z: 35.6237 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj









