[English] 日本語
 Yorodumi
Yorodumi- PDB-5e5u: Crystal structure of the complex between Carbonic anhydrase-like ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5e5u | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the complex between Carbonic anhydrase-like domain of PTPRG and Immunoglobulin domains 2-3 of CNTN6 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | Cell Adhesion/Hydrolase / Neural cell adhesion molecule / Receptor-type protein tyrosine phosphatase / Immunoglobulin domains / Carbonic anhydrase-like domain / Cell Adhesion-Hydrolase complex | ||||||
| Function / homology |  Function and homology information Function and homology informationdendrite self-avoidance / negative regulation of epithelial cell migration / cell-cell adhesion mediator activity / positive regulation of Notch signaling pathway / homophilic cell-cell adhesion / parallel fiber to Purkinje cell synapse / side of membrane / Notch signaling pathway / protein-tyrosine-phosphatase / protein tyrosine phosphatase activity ...dendrite self-avoidance / negative regulation of epithelial cell migration / cell-cell adhesion mediator activity / positive regulation of Notch signaling pathway / homophilic cell-cell adhesion / parallel fiber to Purkinje cell synapse / side of membrane / Notch signaling pathway / protein-tyrosine-phosphatase / protein tyrosine phosphatase activity / axon guidance / synapse organization / neuron projection development / negative regulation of neuron projection development / presynapse / presynaptic membrane / axon / synapse / signal transduction / extracellular space / identical protein binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2 Å molecular replacement / Resolution: 2 Å | ||||||
 Authors Authors | Nikolaienko, R.M. / Bouyain, S. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2016 Journal: J.Biol.Chem. / Year: 2016Title: Structural Basis for Interactions Between Contactin Family Members and Protein-tyrosine Phosphatase Receptor Type G in Neural Tissues. Authors: Nikolaienko, R.M. / Hammel, M. / Dubreuil, V. / Zalmai, R. / Hall, D.R. / Mehzabeen, N. / Karuppan, S.J. / Harroch, S. / Stella, S.L. / Bouyain, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5e5u.cif.gz 5e5u.cif.gz | 395.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5e5u.ent.gz pdb5e5u.ent.gz | 323.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5e5u.json.gz 5e5u.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e5/5e5u https://data.pdbj.org/pub/pdb/validation_reports/e5/5e5u ftp://data.pdbj.org/pub/pdb/validation_reports/e5/5e5u ftp://data.pdbj.org/pub/pdb/validation_reports/e5/5e5u | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5e4iC  5e4qC  5e4sC  5e52C  5e53C  5e55C  5e5rC  5e7lC  5i99C  3kldS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 4 molecules ACBD
| #1: Protein | Mass: 30006.334 Da / Num. of mol.: 2 / Fragment: CA domain, UNP residues 57-320 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Protein | Mass: 22334.201 Da / Num. of mol.: 2 / Fragment: Immunoglobulin domains 2-3, UNP residues 119-316 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 6 types, 549 molecules 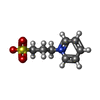

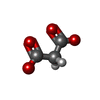

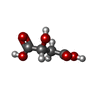






| #3: Chemical | ChemComp-1PS / #4: Chemical | #5: Chemical | ChemComp-MLI / | #6: Chemical | #7: Chemical | ChemComp-MLT / #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 50.71 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7 / Details: 55% (v/v) Tacsimate pH 7.0, 150 mM NDSB 201 |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-BM / Wavelength: 1 Å / Beamline: 22-BM / Wavelength: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Mar 13, 2013 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: ROSENBAUM-ROCK DOUBLE-CRYSTAL MONOCHROMATOR / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2→50 Å / Num. obs: 70482 / % possible obs: 98.7 % / Redundancy: 7.1 % / Biso Wilson estimate: 31.65 Å2 / Rmerge(I) obs: 0.096 / Χ2: 0.958 / Net I/av σ(I): 19.509 / Net I/σ(I): 6.4 / Num. measured all: 500144 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3KLD Resolution: 2→24.891 Å / SU ML: 0.22 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 23.05 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 165.16 Å2 / Biso mean: 40.7988 Å2 / Biso min: 15.15 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2→24.891 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 25
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj
















