[English] 日本語
 Yorodumi
Yorodumi- PDB-4xnc: Crystal structure at room temperature of cyclophilin D in complex... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4xnc | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure at room temperature of cyclophilin D in complex with an inhibitor | ||||||
 Components Components | Peptidyl-prolyl cis-trans isomerase F, mitochondrial | ||||||
 Keywords Keywords | ISOMERASE / inhibitor isomerase fragment based drug design | ||||||
| Function / homology |  Function and homology information Function and homology information: / : / mitochondrial outer membrane permeabilization involved in programmed cell death / regulation of mitochondrial membrane permeability involved in programmed necrotic cell death / skeletal muscle fiber differentiation / mitochondrial permeability transition pore complex / cellular response to arsenic-containing substance / mitochondrial depolarization / negative regulation of ATP-dependent activity / negative regulation of oxidative phosphorylation ...: / : / mitochondrial outer membrane permeabilization involved in programmed cell death / regulation of mitochondrial membrane permeability involved in programmed necrotic cell death / skeletal muscle fiber differentiation / mitochondrial permeability transition pore complex / cellular response to arsenic-containing substance / mitochondrial depolarization / negative regulation of ATP-dependent activity / negative regulation of oxidative phosphorylation / regulation of mitochondrial membrane permeability / cyclosporin A binding / negative regulation of release of cytochrome c from mitochondria / negative regulation of intrinsic apoptotic signaling pathway / necroptotic process / apoptotic mitochondrial changes / cellular response to calcium ion / response to ischemia / peptidylprolyl isomerase / peptidyl-prolyl cis-trans isomerase activity / cellular response to hydrogen peroxide / protein folding / mitochondrial matrix / negative regulation of apoptotic process / mitochondrion / membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.23 Å MOLECULAR REPLACEMENT / Resolution: 2.23 Å | ||||||
 Authors Authors | Gelin, M. / Allemand, F. / Labesse, G. / Guichou, J.F. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2015 Journal: Acta Crystallogr.,Sect.D / Year: 2015Title: Combining `dry' co-crystallization and in situ diffraction to facilitate ligand screening by X-ray crystallography. Authors: Gelin, M. / Delfosse, V. / Allemand, F. / Hoh, F. / Sallaz-Damaz, Y. / Pirocchi, M. / Bourguet, W. / Ferrer, J.L. / Labesse, G. / Guichou, J.F. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4xnc.cif.gz 4xnc.cif.gz | 46.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4xnc.ent.gz pdb4xnc.ent.gz | 31.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4xnc.json.gz 4xnc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xn/4xnc https://data.pdbj.org/pub/pdb/validation_reports/xn/4xnc ftp://data.pdbj.org/pub/pdb/validation_reports/xn/4xnc ftp://data.pdbj.org/pub/pdb/validation_reports/xn/4xnc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3rdcC  4xldC  4xn6C  4xneC  4xoyC  4xozC  4xp0C  4xp2C  4xp3C  4xrjC  4xrlC  4zscC  4zsdC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | biological unit is the same as asym. |
- Components
Components
| #1: Protein | Mass: 17652.125 Da / Num. of mol.: 1 / Fragment: Residues 44-207 / Mutation: K175I Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PPIF, CYP3 / Production host: Homo sapiens (human) / Gene: PPIF, CYP3 / Production host:  |
|---|---|
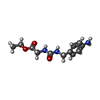
| #2: Chemical | ChemComp-EA4 / |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.11 Å3/Da / Density % sol: 41.6 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, sitting drop / pH: 7.3 / Details: 25% (w/v) PEG4000 |
-Data collection
| Diffraction | Mean temperature: 298 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.542 Å ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.542 Å |
| Detector | Type: ADSC QUANTUM 210 / Detector: CCD / Date: May 12, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.542 Å / Relative weight: 1 |
| Reflection | Resolution: 2.23→29.5 Å / Num. obs: 6820 / % possible obs: 98.1 % / Redundancy: 4.5 % / Net I/σ(I): 18.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.23→29.02 Å / Cor.coef. Fo:Fc: 0.962 / Cor.coef. Fo:Fc free: 0.949 / SU B: 4.578 / SU ML: 0.116 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.307 / ESU R Free: 0.192 / Stereochemistry target values: MAXIMUM LIKELIHOOD MOLECULAR REPLACEMENT / Resolution: 2.23→29.02 Å / Cor.coef. Fo:Fc: 0.962 / Cor.coef. Fo:Fc free: 0.949 / SU B: 4.578 / SU ML: 0.116 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.307 / ESU R Free: 0.192 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 40.42 Å2 / Biso mean: 17.087 Å2 / Biso min: 6.72 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.23→29.02 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.233→2.291 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj