+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4wrl | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the human CSF-1:CSF-1R complex | |||||||||
 Components Components | (Macrophage colony-stimulating factor ...) x 2 | |||||||||
 Keywords Keywords | CYTOKINE/CYTOKINE RECEPTOR / cytokine-cytokine receptor complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of macrophage derived foam cell differentiation / mammary gland fat development / positive regulation of macrophage colony-stimulating factor signaling pathway / monocyte homeostasis / macrophage homeostasis / macrophage colony-stimulating factor receptor binding / positive regulation of macrophage migration / osteoclast proliferation / positive regulation of odontogenesis of dentin-containing tooth / forebrain neuron differentiation ...regulation of macrophage derived foam cell differentiation / mammary gland fat development / positive regulation of macrophage colony-stimulating factor signaling pathway / monocyte homeostasis / macrophage homeostasis / macrophage colony-stimulating factor receptor binding / positive regulation of macrophage migration / osteoclast proliferation / positive regulation of odontogenesis of dentin-containing tooth / forebrain neuron differentiation / developmental process involved in reproduction / macrophage colony-stimulating factor receptor activity / CSF1-CSF1R complex / monocyte activation / macrophage colony-stimulating factor signaling pathway / regulation of macrophage migration / positive regulation of microglial cell migration / mammary duct terminal end bud growth / cellular response to macrophage colony-stimulating factor stimulus / cell-cell junction maintenance / microglial cell proliferation / positive regulation of mononuclear cell proliferation / positive regulation of macrophage differentiation / olfactory bulb development / mammary gland duct morphogenesis / myeloid leukocyte migration / neutrophil homeostasis / host-mediated activation of viral process / positive regulation of monocyte differentiation / ruffle organization / positive regulation of multicellular organism growth / positive regulation of osteoclast differentiation / positive regulation of macrophage proliferation / positive regulation of macrophage derived foam cell differentiation / branching involved in mammary gland duct morphogenesis / positive regulation of Ras protein signal transduction / regulation of bone resorption / positive regulation of cell motility / Other interleukin signaling / positive regulation of tyrosine phosphorylation of STAT protein / growth factor binding / positive regulation of cell-matrix adhesion / positive regulation of protein tyrosine kinase activity / cytokine binding / positive regulation of macrophage chemotaxis / cellular response to cytokine stimulus / Interleukin-10 signaling / positive regulation of protein metabolic process / regulation of MAPK cascade / hemopoiesis / Transcriptional Regulation by VENTX / macrophage differentiation / monocyte differentiation / regulation of ossification / positive regulation of chemokine production / Transcriptional and post-translational regulation of MITF-M expression and activity / homeostasis of number of cells within a tissue / axon guidance / cell surface receptor protein tyrosine kinase signaling pathway / osteoclast differentiation / peptidyl-tyrosine phosphorylation / cytokine activity / response to ischemia / regulation of actin cytoskeleton organization / Post-translational protein phosphorylation / growth factor activity / receptor protein-tyrosine kinase / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / cytokine-mediated signaling pathway / positive regulation of protein phosphorylation / Signaling by CSF1 (M-CSF) in myeloid cells / cell migration / regulation of cell shape / protein autophosphorylation / protein tyrosine kinase activity / protein phosphatase binding / Ras protein signal transduction / positive regulation of ERK1 and ERK2 cascade / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / cell population proliferation / receptor complex / nuclear body / positive regulation of cell migration / endoplasmic reticulum lumen / inflammatory response / negative regulation of cell population proliferation / innate immune response / intracellular membrane-bounded organelle / positive regulation of cell population proliferation / positive regulation of gene expression / negative regulation of apoptotic process / perinuclear region of cytoplasm / cell surface / signal transduction / protein homodimerization activity / extracellular space / extracellular region / nucleoplasm / ATP binding / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.802 Å MOLECULAR REPLACEMENT / Resolution: 2.802 Å | |||||||||
 Authors Authors | Felix, J. / De Munck, S. / Elegheert, J. / Savvides, S.N. | |||||||||
 Citation Citation |  Journal: Structure / Year: 2015 Journal: Structure / Year: 2015Title: Structure and Assembly Mechanism of the Signaling Complex Mediated by Human CSF-1. Authors: Jan Felix / Steven De Munck / Kenneth Verstraete / Leander Meuris / Nico Callewaert / Jonathan Elegheert / Savvas N Savvides /  Abstract: Human colony-stimulating factor 1 receptor (hCSF-1R) is unique among the hematopoietic receptors because it is activated by two distinct cytokines, CSF-1 and interleukin-34 (IL-34). Despite ever- ...Human colony-stimulating factor 1 receptor (hCSF-1R) is unique among the hematopoietic receptors because it is activated by two distinct cytokines, CSF-1 and interleukin-34 (IL-34). Despite ever-growing insights into the central role of hCSF-1R signaling in innate and adaptive immunity, inflammatory diseases, and cancer, the structural basis of the functional dichotomy of hCSF-1R has remained elusive. Here, we report crystal structures of ternary complexes between hCSF-1 and hCSF-1R, including their complete extracellular assembly, and propose a mechanism for the cooperative human CSF-1:CSF-1R complex that relies on the adoption by dimeric hCSF-1 of an active conformational state and homotypic receptor interactions. Furthermore, we trace the cytokine-binding duality of hCSF-1R to a limited set of conserved interactions mediated by functionally equivalent residues on CSF-1 and IL-34 that play into the geometric requirements of hCSF-1R activation, and map the possible mechanistic consequences of somatic mutations in hCSF-1R associated with cancer. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4wrl.cif.gz 4wrl.cif.gz | 359 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4wrl.ent.gz pdb4wrl.ent.gz | 294.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4wrl.json.gz 4wrl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wr/4wrl https://data.pdbj.org/pub/pdb/validation_reports/wr/4wrl ftp://data.pdbj.org/pub/pdb/validation_reports/wr/4wrl ftp://data.pdbj.org/pub/pdb/validation_reports/wr/4wrl | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4wrmC  3uf2S  4dkdS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Macrophage colony-stimulating factor ... , 2 types, 4 molecules ACBD
| #1: Protein | Mass: 31583.867 Da / Num. of mol.: 2 / Fragment: UNP residues 20-296 / Mutation: N240Q Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CSF1R, FMS / Cell line (production host): HEK-293S / Production host: Homo sapiens (human) / Gene: CSF1R, FMS / Cell line (production host): HEK-293S / Production host:  Homo sapiens (human) Homo sapiens (human)References: UniProt: P07333, receptor protein-tyrosine kinase #2: Protein | Mass: 19757.314 Da / Num. of mol.: 2 / Fragment: UNP residues 33-181 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CSF1 / Production host: Homo sapiens (human) / Gene: CSF1 / Production host:  |
|---|
-Sugars , 5 types, 9 molecules 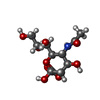


| #3: Polysaccharide | | #4: Polysaccharide | beta-D-galactopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #5: Polysaccharide | #6: Sugar | ChemComp-SIA / | #7: Sugar | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.44 Å3/Da / Density % sol: 64.28 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 0.2 M Lithium sulfate monohydrate, 0.1 M Tris pH 8.5 and 28% w/v PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 0.97625 Å / Beamline: ID23-1 / Wavelength: 0.97625 Å |
| Detector | Type: PSI PILATUS 6M / Detector: PIXEL / Date: Apr 25, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97625 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→49.71 Å / Num. obs: 34125 / % possible obs: 99.8 % / Redundancy: 6.9 % / Biso Wilson estimate: 82.26 Å2 / Rmerge(I) obs: 0.064 / Net I/σ(I): 20.73 |
| Reflection shell | Resolution: 2.8→2.97 Å / Redundancy: 6.8 % / Rmerge(I) obs: 0.815 / Mean I/σ(I) obs: 2.32 / % possible all: 99.2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4DKD, 3UF2 Resolution: 2.802→49.71 Å / SU ML: 0.52 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 30.59 / Stereochemistry target values: ML Details: Galactose 403 and Galactose 408 on chain C failed to be fitted in the exo-anomeric conformation of their glycosidic bond
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 1 Å / VDW probe radii: 1.2 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.802→49.71 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj