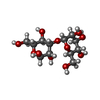
Entry Database : PDB / ID : 4v28Title Structure of an E333Q variant of the GH99 endo-alpha-mannanase from Bacteroides xylanisolvens in complex with Man-Man-Methylumbelliferone GLYCOSYL HYDROLASE FAMILY 71 Keywords / / / / / / / Function / homology Function Domain/homology Component
Biological species BACTEROIDES XYLANISOLVENS (bacteria)Method / / / Resolution : 1.2 Å Authors Hakki, Z. / Bellmaine, S. / Thompson, A.J. / Speciale, G. / Davies, G.J. / Williams, S.J. Journal : Chemistry / Year : 2015Title : Structural and Kinetic Dissection of the Endo-Alpha-1,2-Mannanase Activity of Bacterial Gh99 Glycoside Hydrolases from Bacteroides Spp.Authors : Hakki, Z. / Thompson, A.J. / Bellmaine, S. / Speciale, G. / Davies, G.J. / Williams, S.J. History Deposition Oct 7, 2014 Deposition site / Processing site Revision 1.0 Dec 24, 2014 Provider / Type Revision 1.1 Feb 4, 2015 Group Revision 2.0 Jul 29, 2020 Group Atomic model / Data collection ... Atomic model / Data collection / Derived calculations / Other / Structure summary Category atom_site / atom_site_anisotrop ... atom_site / atom_site_anisotrop / chem_comp / entity / entity_name_com / pdbx_branch_scheme / pdbx_chem_comp_identifier / pdbx_database_status / pdbx_entity_branch / pdbx_entity_branch_descriptor / pdbx_entity_branch_link / pdbx_entity_branch_list / pdbx_entity_nonpoly / pdbx_molecule_features / pdbx_nonpoly_scheme / pdbx_struct_assembly_gen / pdbx_struct_special_symmetry / struct_asym / struct_conn / struct_site / struct_site_gen Item _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.type_symbol / _atom_site_anisotrop.U[1][1] / _atom_site_anisotrop.U[1][2] / _atom_site_anisotrop.U[1][3] / _atom_site_anisotrop.U[2][2] / _atom_site_anisotrop.U[2][3] / _atom_site_anisotrop.U[3][3] / _atom_site_anisotrop.pdbx_auth_asym_id / _atom_site_anisotrop.pdbx_auth_atom_id / _atom_site_anisotrop.pdbx_auth_comp_id / _atom_site_anisotrop.pdbx_auth_seq_id / _atom_site_anisotrop.pdbx_label_asym_id / _atom_site_anisotrop.pdbx_label_atom_id / _atom_site_anisotrop.pdbx_label_comp_id / _atom_site_anisotrop.type_symbol / _chem_comp.name / _chem_comp.type / _entity.formula_weight / _entity.pdbx_description / _entity.pdbx_number_of_molecules / _entity.src_method / _entity.type / _pdbx_database_status.status_code_sf / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_special_symmetry.label_asym_id / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id Description / Provider / Type Revision 2.1 Jan 10, 2024 Group Data collection / Database references ... Data collection / Database references / Refinement description / Structure summary Category chem_comp / chem_comp_atom ... chem_comp / chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_DOI / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information BACTEROIDES XYLANISOLVENS (bacteria)
BACTEROIDES XYLANISOLVENS (bacteria) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.2 Å
MOLECULAR REPLACEMENT / Resolution: 1.2 Å  Authors
Authors Citation
Citation Journal: Chemistry / Year: 2015
Journal: Chemistry / Year: 2015 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4v28.cif.gz
4v28.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4v28.ent.gz
pdb4v28.ent.gz PDB format
PDB format 4v28.json.gz
4v28.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/v2/4v28
https://data.pdbj.org/pub/pdb/validation_reports/v2/4v28 ftp://data.pdbj.org/pub/pdb/validation_reports/v2/4v28
ftp://data.pdbj.org/pub/pdb/validation_reports/v2/4v28

 Links
Links Assembly
Assembly
 Components
Components BACTEROIDES XYLANISOLVENS (bacteria) / Strain: XB1A / Plasmid: PET28A / Production host:
BACTEROIDES XYLANISOLVENS (bacteria) / Strain: XB1A / Plasmid: PET28A / Production host: 






 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I03 / Wavelength: 0.9762
/ Beamline: I03 / Wavelength: 0.9762  Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller














 PDBj
PDBj