[English] 日本語
 Yorodumi
Yorodumi- PDB-4uxg: Crystal structure of the carboxy-terminal region of the bacteriop... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4uxg | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the carboxy-terminal region of the bacteriophage T4 proximal long tail fibre protein gp34, R32 native crystal | ||||||
 Components Components | LARGE TAIL FIBER PROTEIN P34 | ||||||
 Keywords Keywords | VIRAL PROTEIN / CAUDOVIRALES / MYOVIRIDAE / TRIPLE BETA-HELIX | ||||||
| Function / homology | : / Long-tail fiber proximal subunit, C-terminal, second / : / Long-tail fiber proximal subunit, C-terminal, trimerization domain / virus tail, fiber / Long-tail fiber proximal subunit Function and homology information Function and homology information | ||||||
| Biological species |  ENTEROBACTERIA PHAGE T4 (virus) ENTEROBACTERIA PHAGE T4 (virus) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3 Å MOLECULAR REPLACEMENT / Resolution: 3 Å | ||||||
 Authors Authors | Granell, M. / Alvira, S. / Garcia-Doval, C. / Singh, A.K. / van Raaij, M.J. | ||||||
 Citation Citation |  Journal: Viruses / Year: 2017 Journal: Viruses / Year: 2017Title: Crystal Structure of the Carboxy-Terminal Region of the Bacteriophage T4 Proximal Long Tail Fiber Protein Gp34. Authors: Granell, M. / Namura, M. / Alvira, S. / Kanamaru, S. / van Raaij, M.J. #1: Journal: Acta Crystallogr.,Sect.F / Year: 2014 Title: Crystallization of the Carboxy-Terminal Region of the Bacteriophage T4 Proximal Long Tail Fibre Protein Gp34. Authors: Granell, M. / Namura, M. / Alvira, S. / Garcia-Doval, C. / Singh, A.K. / Gutsche, I. / Van Raaij, M.J. / Kanamaru, S. | ||||||
| History |
| ||||||
| Remark 700 | SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN ... SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE SHEET RECORDS BELOW, TWO SHEETS ARE DEFINED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4uxg.cif.gz 4uxg.cif.gz | 875.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4uxg.ent.gz pdb4uxg.ent.gz | 731.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4uxg.json.gz 4uxg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ux/4uxg https://data.pdbj.org/pub/pdb/validation_reports/ux/4uxg ftp://data.pdbj.org/pub/pdb/validation_reports/ux/4uxg ftp://data.pdbj.org/pub/pdb/validation_reports/ux/4uxg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4uxeC  4uxfSC  5nxfC  5nxhC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 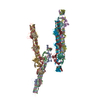
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 44559.973 Da / Num. of mol.: 12 / Fragment: CARBOXY-TERMINAL REGION, RESIDUES 894-1289 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  ENTEROBACTERIA PHAGE T4 (virus) ENTEROBACTERIA PHAGE T4 (virus)Description: GERMAN COLLECTION OF MICROORGANISMS (DSMZ), DSM 4505 Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 5 Å3/Da / Density % sol: 76 % / Description: NONE |
|---|---|
| Crystal grow | pH: 8.5 Details: 1.0-1.2 M AMMONIUM SULFATE, 6-16% (V/V) GLYCEROL, 0.1 M TRIS-HCL PH 8.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-4 / Wavelength: 0.9393 / Beamline: ID14-4 / Wavelength: 0.9393 |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Jun 30, 2012 / Details: TOROIDAL FOCUSING MIRROR |
| Radiation | Monochromator: CHANNEL CUT ESRF MONOCHROMATOR SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9393 Å / Relative weight: 1 |
| Reflection | Resolution: 3→30 Å / Num. obs: 214066 / % possible obs: 99.8 % / Redundancy: 4.6 % / Biso Wilson estimate: 55.6 Å2 / Rmerge(I) obs: 0.13 / Net I/σ(I): 8.4 |
| Reflection shell | Resolution: 3→3.16 Å / Redundancy: 4.7 % / Rmerge(I) obs: 0.36 / Mean I/σ(I) obs: 3.5 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4UXF Resolution: 3→29.986 Å / SU ML: 0.38 / σ(F): 1.36 / Phase error: 27.79 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 51.4 Å2 | ||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3→29.986 Å
| ||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj