[English] 日本語
 Yorodumi
Yorodumi- PDB-4ubp: STRUCTURE OF BACILLUS PASTEURII UREASE INHIBITED WITH ACETOHYDROX... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4ubp | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF BACILLUS PASTEURII UREASE INHIBITED WITH ACETOHYDROXAMIC ACID AT 1.55 A RESOLUTION | ||||||
 Components Components | (PROTEIN (UREASE (CHAIN ...) x 3 | ||||||
 Keywords Keywords | HYDROLASE / UREASE / BACILLUS PASTEURII / NICKEL / ACETOHYDROXAMIC ACID / METALLOENZYME | ||||||
| Function / homology |  Function and homology information Function and homology informationurease complex / urease / urease activity / urea catabolic process / nickel cation binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  Sporosarcina pasteurii (bacteria) Sporosarcina pasteurii (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.55 Å MOLECULAR REPLACEMENT / Resolution: 1.55 Å | ||||||
 Authors Authors | Benini, S. / Rypniewski, W.R. / Wilson, K.S. / Ciurli, S. / Mangani, S. | ||||||
 Citation Citation |  Journal: J.Biol.Inorg.Chem. / Year: 2000 Journal: J.Biol.Inorg.Chem. / Year: 2000Title: The complex of Bacillus pasteurii urease with acetohydroxamate anion from X-ray data at 1.55 A resolution. Authors: Benini, S. / Rypniewski, W.R. / Wilson, K.S. / Miletti, S. / Ciurli, S. / Mangani, S. #1:  Journal: Structure / Year: 1999 Journal: Structure / Year: 1999Title: A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Authors: Benini, S. / Rypniewski, W.R. / Wilson, K.S. / Miletti, S. / Ciurli, S. / Mangani, S. #2: Journal: Acta Crystallogr.,Sect.D / Year: 1998 Title: Crystallization and preliminary high-resolution X-ray diffraction analysis of native and beta-mercaptoethanol-inhibited urease from Bacillus pasteurii. Authors: Benini, S. / Ciurli, S. / Rypniewski, W.R. / Wilson, K.S. / Mangani, S. #3:  Journal: J.Biol.Inorg.Chem. / Year: 1998 Journal: J.Biol.Inorg.Chem. / Year: 1998Title: The Complex of Bacillus pasteurii Urease with Beta-Mercaptoethanol from X-Ray Data at 1.65 A Resolution Authors: Benini, S. / Rypniewski, W.R. / Wilson, K.S. / Ciurli, S. / Mangani, S. #4:  Journal: Soil Biol.Biochem. / Year: 1996 Journal: Soil Biol.Biochem. / Year: 1996Title: Bacillus pasteurii Urease: A Heteropolimeric Enzyme with a Binuclear Nickel Active Site Authors: Benini, S. / Gessa, C. / Ciurli, S. #5: Journal: Eur.J.Biochem. / Year: 1996 Title: X-ray absorption spectroscopy study of native and phenylphosphorodiamidate-inhibited Bacillus pasteurii urease. Authors: Benini, S. / Ciurli, S. / Nolting, H.F. / Mangani, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4ubp.cif.gz 4ubp.cif.gz | 186.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4ubp.ent.gz pdb4ubp.ent.gz | 142.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4ubp.json.gz 4ubp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ub/4ubp https://data.pdbj.org/pub/pdb/validation_reports/ub/4ubp ftp://data.pdbj.org/pub/pdb/validation_reports/ub/4ubp ftp://data.pdbj.org/pub/pdb/validation_reports/ub/4ubp | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2ubpS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-PROTEIN (UREASE (CHAIN ... , 3 types, 3 molecules ABC
| #1: Protein | Mass: 11187.017 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Sporosarcina pasteurii (bacteria) / Cellular location: CYTOPLASM / Strain: DSM 33 / References: UniProt: P41022, urease Sporosarcina pasteurii (bacteria) / Cellular location: CYTOPLASM / Strain: DSM 33 / References: UniProt: P41022, urease |
|---|---|
| #2: Protein | Mass: 13975.539 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Sporosarcina pasteurii (bacteria) / Cellular location: CYTOPLASM / Strain: DSM 33 / References: UniProt: P41021, urease Sporosarcina pasteurii (bacteria) / Cellular location: CYTOPLASM / Strain: DSM 33 / References: UniProt: P41021, urease |
| #3: Protein | Mass: 61646.766 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Sporosarcina pasteurii (bacteria) / Cellular location: CYTOPLASM / Strain: DSM 33 / References: UniProt: P41020, urease Sporosarcina pasteurii (bacteria) / Cellular location: CYTOPLASM / Strain: DSM 33 / References: UniProt: P41020, urease |
-Non-polymers , 3 types, 749 molecules 
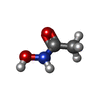



| #4: Chemical | | #5: Chemical | ChemComp-HAE / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.7 Å3/Da / Density % sol: 54 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 6.3 Details: ATED AMMONIUM SULPHATE, 1.2 M LICL), DROXAMIC ACID, 1OOMM SODIUM CITRATE PH 6.3. HANGING DROP 20 C, 3 UL PROTEIN SOLUTION (11 MG/ML IN 20 MM TRIS HCL PH 8.0 + 4MM ACETOHYDROXAMIC ACID) + 3 ...Details: ATED AMMONIUM SULPHATE, 1.2 M LICL), DROXAMIC ACID, 1OOMM SODIUM CITRATE PH 6.3. HANGING DROP 20 C, 3 UL PROTEIN SOLUTION (11 MG/ML IN 20 MM TRIS HCL PH 8.0 + 4MM ACETOHYDROXAMIC ACID) + 3 MICROLITERS PRECIPITANT SOLUTION, pH 6.30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal | *PLUS Density % sol: 54 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / Method: vapor diffusion, hanging drop / pH: 7.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: BW7B / Wavelength: 0.8342 / Beamline: BW7B / Wavelength: 0.8342 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Apr 30, 1998 / Details: BENT MIRROR |
| Radiation | Monochromator: SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.8342 Å / Relative weight: 1 |
| Reflection | Resolution: 1.55→35 Å / Num. obs: 138830 / % possible obs: 99.5 % / Observed criterion σ(I): -3 / Redundancy: 26.02 % / Rmerge(I) obs: 0.071 / Rsym value: 0.071 / Net I/σ(I): 15.03 |
| Reflection shell | Resolution: 1.55→1.58 Å / Redundancy: 4.29 % / Rmerge(I) obs: 0.479 / Mean I/σ(I) obs: 2.23 / Rsym value: 0.479 / % possible all: 99.9 |
| Reflection | *PLUS Highest resolution: 1.55 Å / Lowest resolution: 32.5 Å / % possible obs: 99.5 % / Num. measured all: 3612270 |
| Reflection shell | *PLUS Highest resolution: 1.55 Å / Lowest resolution: 1.58 Å / % possible obs: 99.5 % / Mean I/σ(I) obs: 2.2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2UBP Resolution: 1.55→32.73 Å / Cross valid method: RFREE / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 26.22 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.55→32.73 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: REFMAC / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.151 / Rfactor Rwork: 0.151 / Highest resolution: 1.55 Å / Lowest resolution: 32.7 Å / Rfactor Rfree: 0.19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller














 PDBj
PDBj



