+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4r2a | ||||||
|---|---|---|---|---|---|---|---|
| Title | Egr1/Zif268 zinc fingers in complex with methylated DNA | ||||||
 Components Components |
| ||||||
 Keywords Keywords | DNA Binding Protein/DNA / Zinc finger / Transcription / DNA Binding Protein-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of protein sumoylation / glomerular mesangial cell proliferation / positive regulation of glomerular metanephric mesangial cell proliferation / cellular response to interleukin-8 / regulation of progesterone biosynthetic process / cellular response to heparin / cellular response to mycophenolic acid / circadian temperature homeostasis / positive regulation of post-translational protein modification / positive regulation of hormone biosynthetic process ...regulation of protein sumoylation / glomerular mesangial cell proliferation / positive regulation of glomerular metanephric mesangial cell proliferation / cellular response to interleukin-8 / regulation of progesterone biosynthetic process / cellular response to heparin / cellular response to mycophenolic acid / circadian temperature homeostasis / positive regulation of post-translational protein modification / positive regulation of hormone biosynthetic process / double-stranded methylated DNA binding / hemi-methylated DNA-binding / positive regulation of gene expression via chromosomal CpG island demethylation / NGF-stimulated transcription / interleukin-1-mediated signaling pathway / histone acetyltransferase binding / locomotor rhythm / T cell differentiation / skeletal muscle cell differentiation / BMP signaling pathway / response to glucose / estrous cycle / positive regulation of chemokine production / regulation of neuron apoptotic process / Regulation of PTEN gene transcription / response to ischemia / positive regulation of interleukin-1 beta production / RNA polymerase II transcription regulatory region sequence-specific DNA binding / circadian regulation of gene expression / promoter-specific chromatin binding / negative regulation of canonical Wnt signaling pathway / cellular response to gamma radiation / response to insulin / positive regulation of miRNA transcription / sequence-specific double-stranded DNA binding / Interferon alpha/beta signaling / DNA-binding transcription activator activity, RNA polymerase II-specific / sequence-specific DNA binding / DNA-binding transcription factor activity, RNA polymerase II-specific / response to hypoxia / transcription cis-regulatory region binding / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / positive regulation of gene expression / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / chromatin / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / DNA binding / zinc ion binding / nucleoplasm / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.591 Å MOLECULAR REPLACEMENT / Resolution: 1.591 Å | ||||||
 Authors Authors | Hashimoto, H. / Olanrewaju, Y.O. / Zheng, Y. / Wilson, G.G. / Zhang, X. / Cheng, X. | ||||||
 Citation Citation |  Journal: Genes Dev. / Year: 2014 Journal: Genes Dev. / Year: 2014Title: Wilms tumor protein recognizes 5-carboxylcytosine within a specific DNA sequence. Authors: Hashimoto, H. / Olanrewaju, Y.O. / Zheng, Y. / Wilson, G.G. / Zhang, X. / Cheng, X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4r2a.cif.gz 4r2a.cif.gz | 84.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4r2a.ent.gz pdb4r2a.ent.gz | 60 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4r2a.json.gz 4r2a.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/r2/4r2a https://data.pdbj.org/pub/pdb/validation_reports/r2/4r2a ftp://data.pdbj.org/pub/pdb/validation_reports/r2/4r2a ftp://data.pdbj.org/pub/pdb/validation_reports/r2/4r2a | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4r2cC  4r2dC  4r2eC  4r2pC  4r2qC  4r2rC  4r2sC  1aayS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 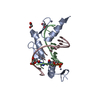
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 11133.757 Da / Num. of mol.: 1 / Fragment: Zinc Finger 1-3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: EGR1, KROX24, ZNF225 / Plasmid: pGEX6p-1, pXC1272 / Production host: Homo sapiens (human) / Gene: EGR1, KROX24, ZNF225 / Plasmid: pGEX6p-1, pXC1272 / Production host:  |
|---|
-DNA chain , 2 types, 2 molecules BC
| #2: DNA chain | Mass: 3444.260 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: chemical synthesis / Source: (synth.) synthetic construct (others) |
|---|---|
| #3: DNA chain | Mass: 3293.178 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: chemical synthesis / Source: (synth.) synthetic construct (others) |
-Non-polymers , 3 types, 187 molecules 




| #4: Chemical | | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.25 Å3/Da / Density % sol: 45.38 % |
|---|---|
| Crystal grow | Temperature: 289 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 12% (v/v) PEG 3350, 4% Tacsimate, pH 7.0, VAPOR DIFFUSION, SITTING DROP, temperature 289K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-BM / Wavelength: 1 Å / Beamline: 22-BM / Wavelength: 1 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Oct 7, 2013 |
| Radiation | Monochromator: Si (111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.59→28.104 Å / Num. obs: 21919 / % possible obs: 99.1 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Redundancy: 7.3 % / Biso Wilson estimate: 17.9 Å2 / Rmerge(I) obs: 0.053 / Net I/σ(I): 28.895 |
| Reflection shell | Resolution: 1.59→1.65 Å / Redundancy: 5.6 % / Rmerge(I) obs: 0.386 / Mean I/σ(I) obs: 3.6 / Num. unique all: 2098 / % possible all: 95.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 1AAY Resolution: 1.591→28.104 Å / SU ML: 0.13 / σ(F): 1.36 / Phase error: 18.12 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.591→28.104 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION
|
 Movie
Movie Controller
Controller













 PDBj
PDBj











































