[English] 日本語
 Yorodumi
Yorodumi- PDB-4jwi: Crystal structure of the substrate binding domain of E.coli DnaK ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4jwi | ||||||
|---|---|---|---|---|---|---|---|
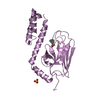
| Title | Crystal structure of the substrate binding domain of E.coli DnaK in complex with sheep Bac7(35-43) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | CHAPERONE/ANTIBIOTIC / chaperone / peptide binding / antimicrobial peptide / CHAPERONE-ANTIBIOTIC complex | ||||||
| Function / homology |  Function and homology information Function and homology informationstress response to copper ion / sigma factor antagonist activity / : / protein unfolding / cellular response to unfolded protein / heat shock protein binding / inclusion body / protein folding chaperone / ATP-dependent protein folding chaperone / lipopolysaccharide binding ...stress response to copper ion / sigma factor antagonist activity / : / protein unfolding / cellular response to unfolded protein / heat shock protein binding / inclusion body / protein folding chaperone / ATP-dependent protein folding chaperone / lipopolysaccharide binding / ADP binding / antimicrobial humoral immune response mediated by antimicrobial peptide / unfolded protein binding / protein-folding chaperone binding / response to heat / protein refolding / protein-containing complex assembly / defense response to Gram-negative bacterium / DNA replication / defense response to Gram-positive bacterium / innate immune response / ATP hydrolysis activity / protein-containing complex / extracellular space / zinc ion binding / ATP binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |   | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 1.9 Å FOURIER SYNTHESIS / Resolution: 1.9 Å | ||||||
 Authors Authors | Zahn, M. / Straeter, N. | ||||||
 Citation Citation |  Journal: Protein Pept.Lett. / Year: 2014 Journal: Protein Pept.Lett. / Year: 2014Title: Structural Identification of DnaK Binding Sites within Bovine and Sheep Bactenecin Bac7. Authors: Zahn, M. / Kieslich, B. / Berthold, N. / Knappe, D. / Hoffmann, R. / Strater, N. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4jwi.cif.gz 4jwi.cif.gz | 187.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4jwi.ent.gz pdb4jwi.ent.gz | 149.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4jwi.json.gz 4jwi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jw/4jwi https://data.pdbj.org/pub/pdb/validation_reports/jw/4jwi ftp://data.pdbj.org/pub/pdb/validation_reports/jw/4jwi ftp://data.pdbj.org/pub/pdb/validation_reports/jw/4jwi | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4jwcC  4jwdC  4jweC  3dpoS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| 2 | 
| |||||||||
| 3 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 23820.777 Da / Num. of mol.: 2 / Fragment: unp residues 389-607 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Protein/peptide | Mass: 1147.438 Da / Num. of mol.: 2 / Fragment: unp residues 165-173 / Source method: obtained synthetically / Source: (synth.)  #3: Chemical | ChemComp-SO4 / #4: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.82 Å3/Da / Density % sol: 56.35 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 4.4 Details: 2.2 M ammonium sulfate, 0.1 M citric acid, pH 4.4, VAPOR DIFFUSION, HANGING DROP, temperature 292K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.1 / Wavelength: 0.918 Å / Beamline: 14.1 / Wavelength: 0.918 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Jul 27, 2011 |
| Radiation | Monochromator: Si 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.918 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→23.79 Å / Num. obs: 45783 / % possible obs: 99.9 % / Biso Wilson estimate: 25.5 Å2 / Rmerge(I) obs: 0.065 |
| Reflection shell | Resolution: 1.9→2 Å / Rmerge(I) obs: 0.414 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: pdb entry 3DPO Resolution: 1.9→23.79 Å / Cor.coef. Fo:Fc: 0.9345 / Cor.coef. Fo:Fc free: 0.9106 / Cross valid method: THROUGHOUT / σ(F): 0
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 34.44 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.259 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→23.79 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.9→1.95 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller

























 PDBj
PDBj