+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4i6s | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of RSL mutant W76A in complex with L-fucose | ||||||
 Components Components | Putative fucose-binding lectin protein | ||||||
 Keywords Keywords | SUGAR BINDING PROTEIN / lectin / beta-propeller / L-fucose / multivalency / Trivalent Fucose binding lectin / Fucosylated oligosaccharides binding / Soluble | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Ralstonia solanacearum (bacteria) Ralstonia solanacearum (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.54 Å MOLECULAR REPLACEMENT / Resolution: 1.54 Å | ||||||
 Authors Authors | Audfray, A. / Arnaud, J. / Varrot, A. / Imberty, A. | ||||||
 Citation Citation |  Journal: Acs Chem.Biol. / Year: 2013 Journal: Acs Chem.Biol. / Year: 2013Title: Reduction of lectin valency drastically changes glycolipid dynamics in membranes but not surface avidity Authors: Arnaud, J. / Claudinon, J. / Trondle, K. / Trovaslet, M. / Larson, G. / Thomas, A. / Varrot, A. / Romer, W. / Imberty, A. / Audfray, A. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: AUTHOR DETERMINED |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4i6s.cif.gz 4i6s.cif.gz | 122.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4i6s.ent.gz pdb4i6s.ent.gz | 95.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4i6s.json.gz 4i6s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i6/4i6s https://data.pdbj.org/pub/pdb/validation_reports/i6/4i6s ftp://data.pdbj.org/pub/pdb/validation_reports/i6/4i6s ftp://data.pdbj.org/pub/pdb/validation_reports/i6/4i6s | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3zi8C  2bt9S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||
| Components on special symmetry positions |
| ||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 9749.626 Da / Num. of mol.: 3 / Mutation: W76A Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Ralstonia solanacearum (bacteria) / Strain: ATCC 11696 / Gene: CMR15_11270, rsc2107 / Plasmid: pET25 / Production host: Ralstonia solanacearum (bacteria) / Strain: ATCC 11696 / Gene: CMR15_11270, rsc2107 / Plasmid: pET25 / Production host:  |
|---|
-Sugars , 2 types, 9 molecules 
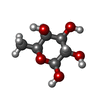

| #2: Sugar | ChemComp-FUC / #3: Sugar | ChemComp-FUL / |
|---|
-Non-polymers , 3 types, 288 molecules 




| #4: Chemical | ChemComp-EDO / | ||
|---|---|---|---|
| #5: Chemical | | #6: Water | ChemComp-HOH / | |
-Details
| Nonpolymer details | HETEROGEN FUC(102 CHAIN A) AND FUL(103 CHAIN A) ARE IN ALTERNATE CONFORMATIONS OF EACH OTHER. ...HETEROGEN FUC(102 CHAIN A) AND FUL(103 CHAIN A) ARE IN ALTERNATE CONFORMATI |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 46.98 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 0.1M Tris/Bicine, 0.12M Monosaccharides, 30% P550MME/P20K, pH 8.5, VAPOR DIFFUSION, HANGING DROP, temperature 292.0K |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.9817 Å / Beamline: PROXIMA 1 / Wavelength: 0.9817 Å | |||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: May 4, 2012 Details: KIRKPATRICK-BAEZ PAIR OF BI-MORPH MIRRORS PLUS CHANNEL CUT CRYOGENICALLY COOLED MONOCHROMATOR CRYSTAL | |||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9817 Å / Relative weight: 1 | |||||||||||||||||||||
| Reflection | Resolution: 1.54→51.88 Å / Num. obs: 41015 / % possible obs: 99.8 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 2 / Redundancy: 9 % / Biso Wilson estimate: 20 Å2 / Rmerge(I) obs: 0.042 / Rsym value: 0.042 / Net I/σ(I): 24.5 | |||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2BT9 Resolution: 1.54→51.88 Å / Cor.coef. Fo:Fc: 0.973 / Cor.coef. Fo:Fc free: 0.969 / SU B: 2.104 / SU ML: 0.041 / Cross valid method: THROUGHOUT / σ(F): 2 / σ(I): 2 / ESU R: 0.067 / ESU R Free: 0.067 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 24.716 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.54→51.88 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.54→1.576 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller









 PDBj
PDBj




