Software Name Version Classification data collectiondata collectionphasingphasingphasingmodel buildingphasing(phenix.refine: 1.8.1_1161)refinementdata reductiondata scalingphasing
Refinement Method to determine structure / Resolution / SU ML / Isotropic thermal model / Cross valid method / σ(F) / Phase error / Stereochemistry target values Rfactor Num. reflection % reflection Selection details Rfree 0.202 1095 5.11 % random Rwork 0.178 - - - all 0.179 21417 - - obs 0.179 21417 95.61 % -
Solvent computation Shrinkage radii / VDW probe radii / Solvent model Displacement parameters Biso mean 2 Refinement step Cycle / Resolution Protein Nucleic acid Ligand Solvent Total Num. atoms 1405 0 6 115 1526
Refine LS restraints Refine-ID Type Dev ideal Number X-RAY DIFFRACTION f_bond_d0.007 1477 X-RAY DIFFRACTION f_angle_d1.132 1995 X-RAY DIFFRACTION f_dihedral_angle_d15.053 577 X-RAY DIFFRACTION f_chiral_restr0.075 241 X-RAY DIFFRACTION f_plane_restr0.005 247
LS refinement shell Refine-ID
Resolution (Å)Rfactor Rfree Num. reflection Rfree Rfactor Rwork Num. reflection Rwork Num. reflection obs % reflection obs (%)1.6296-1.7037 0.3018 126 0.2872 2375 2501 91 1.7037-1.7935 0.2861 129 0.2465 2553 2682 96 1.7935-1.9059 0.257 120 0.2169 2554 2674 96 1.9059-2.0529 0.2037 139 0.1957 2572 2711 97 2.0529-2.2594 0.2291 145 0.1926 2569 2714 97 2.2594-2.586 0.2283 141 0.1981 2588 2729 98 2.586-3.2569 0.1964 152 0.182 2600 2752 97 3.2569-24.5237 0.175 143 0.1479 2511 2654 92
Refinement TLS params. Method / Refine-ID
Show large table (25 x 18) Hide large table ID L11 (°2 )L12 (°2 )L13 (°2 )L22 (°2 )L23 (°2 )L33 (°2 )S11 (Å °)S12 (Å °)S13 (Å °)S21 (Å °)S22 (Å °)S23 (Å °)S31 (Å °)S32 (Å °)S33 (Å °)T11 (Å2 )T12 (Å2 )T13 (Å2 )T22 (Å2 )T23 (Å2 )T33 (Å2 )Origin x (Å)Origin y (Å)Origin z (Å)1 6.1831 0.5575 0.882 5.0706 0.035 5.8353 -0.6617 0.8967 -0.1014 -0.7428 0.4956 -0.6839 0.4566 0.6942 -0.0406 0.4083 0.066 0.0248 0.3483 -0.0596 0.3395 25.0318 14.4892 -2.9435 2 4.0696 0.4502 2.1314 3.7977 -0.3922 4.8058 0.3627 0.7472 -0.63 0.2023 -0.293 -0.0207 1.702 0.0245 -0.1298 0.5301 -0.0416 -0.0015 0.3648 -0.0597 0.276 15.1358 10.1617 -0.8672 3 3.9399 -0.493 0.9453 3.4076 -0.2073 4.897 0.2539 -0.317 -0.7304 -0.0371 -0.1353 -0.6989 1.4773 0.1646 -0.0698 0.375 0.1544 0.0204 0.2929 -0.0909 0.3527 27.3581 12.6698 6.2563 4 5.8373 -0.0045 1.2222 4.3515 -0.9496 6.4552 -0.1075 0.6092 -0.2183 -0.0651 0.0804 -0.9934 -0.2112 0.8772 -0.0123 0.3331 0.0312 0.0189 0.3297 0.0127 0.4318 28.9138 20.1848 1.7851 5 2.2757 -1.661 -0.9154 5.9755 1.0371 9.1024 0.2759 1.3223 0.1924 -0.3392 -0.3231 0.1871 0.2937 -1.3572 0.1288 0.5035 -0.0376 -0.0379 0.5685 -0.0291 0.2756 11.0522 15.6334 -7.0999 6 4.6271 0.0746 2.1271 3.4585 1.3773 6.881 -0.1456 -0.0876 0.1861 -0.149 0.0286 0.0865 -0.3035 -0.4196 0.1265 0.2738 0.0108 0.0206 0.2274 0.0079 0.2062 17.9101 21.9784 5.7125 7 6.416 1.491 3.2324 3.1523 1.7249 7.0854 -0.23 0.73 0.9156 -0.587 -0.0621 -0.0568 0.011 0.1912 0.1286 0.418 0.0385 0.0461 0.4034 0.0741 0.3467 21.1158 22.6663 -4.9312 8 4.9578 0.114 0.291 4.1485 -1.2036 4.9379 -0.2576 -0.6501 -0.2592 0.5539 0.089 -0.7051 -0.0572 0.2421 0.1505 0.4194 0.0659 -0.0821 0.3368 -0.0179 0.337 24.6676 19.1633 12.6696 9 5.4185 0.8797 -0.0313 7.0222 -0.6959 4.3251 -0.1082 0.4708 -0.08 -0.0724 -0.0894 -0.0029 0.4465 -0.4188 0.0781 0.3643 -0.07 -0.0059 0.3101 -0.0304 0.1963 9.4596 15.463 4.2178 10 6.2269 -2.507 3.7272 1.0456 -1.5333 2.3408 0.4218 -1.1127 -0.7196 0.5092 -0.214 -0.4833 0.846 -0.176 0.1643 0.4066 0.0365 -0.0386 0.3546 -0.0128 0.3209 25.1113 13.3302 11.2395 11 6.3874 4.352 -5.8404 5.5087 -5.4382 6.9053 0.391 -0.5969 0.5791 0.4516 0.0654 0.8007 -0.0965 0.0121 -0.5213 0.3161 0.0608 0.0062 0.4952 0.0373 0.3822 -8.6473 20.3904 24.3386 12 1.9971 0.9395 -0.5425 6.3884 -2.4748 3.1497 0.0353 -0.1495 0.1871 0.5249 0.0727 0.1391 -0.2901 0.0438 -0.0669 0.2076 0.0064 0.0156 0.3242 -0.0066 0.2358 0.6951 20.4438 23.5136 13 3.3454 2.2573 -2.5067 7.9032 -5.2455 4.3503 -0.0563 0.219 0.4106 0.0411 0.3749 0.3808 -0.166 -0.0931 -0.3611 0.2471 -0.0125 -0.0312 0.3746 0.06 0.3479 -4.5135 26.1931 15.249 14 2.3 0.3522 0.1434 6.1806 -2.6922 5.4051 0.1312 0.3105 0.0532 -0.4028 -0.1407 0.0021 0.5359 -0.1755 -0.0607 0.2561 -0.0572 0.0128 0.3223 0.0045 0.2528 2.6673 17.1725 13.0525 15 2.2332 1.4969 -0.6973 4.0051 -4.9437 6.4098 -0.0056 0.2853 0.4606 -0.0966 0.2727 0.4816 -0.4007 -0.326 -0.6478 0.2888 0.0253 -0.0099 0.4964 0.1104 0.3818 -7.6631 22.4564 12.9934 16 0.2272 0.888 -0.6573 5.6151 -3.6279 4.0879 -0.0704 -0.0321 -0.0368 -0.3562 -0.0864 -0.1632 0.2153 0.2443 0.1946 0.2102 0.0204 0.0119 0.2975 -0.0017 0.2511 4.9351 12.1649 21.3111 17 4.308 1.8558 -1.1174 6.1787 -1.4596 5.2433 -0.1246 0.151 0.0724 -0.2995 0.3388 -0.1512 -0.2674 0.0908 -0.0891 0.2301 -0.0219 0.025 0.2545 0.0075 0.2952 8.3462 26.1362 10.7878 18 3.779 2.853 -2.865 3.6079 -4.5031 6.2974 0.0289 -0.5056 -0.3058 0.1893 -0.3662 -0.6417 0.084 0.6109 0.1137 0.2623 -0.0034 -0.0331 0.3338 0.0335 0.3142 5.9327 16.7656 25.0451
Refinement TLS group Show large table (4 x 18) Hide large table ID Refine-ID Refine TLS-ID Selection details 1 X-RAY DIFFRACTION 1 chain 'A' and (resid 43 through 50 )2 X-RAY DIFFRACTION 2 chain 'A' and (resid 51 through 55 )3 X-RAY DIFFRACTION 3 chain 'A' and (resid 56 through 62 )4 X-RAY DIFFRACTION 4 chain 'A' and (resid 63 through 73 )5 X-RAY DIFFRACTION 5 chain 'A' and (resid 74 through 78 )6 X-RAY DIFFRACTION 6 chain 'A' and (resid 79 through 92 )7 X-RAY DIFFRACTION 7 chain 'A' and (resid 93 through 102 )8 X-RAY DIFFRACTION 8 chain 'A' and (resid 103 through 113 )9 X-RAY DIFFRACTION 9 chain 'A' and (resid 114 through 122 )10 X-RAY DIFFRACTION 10 chain 'A' and (resid 123 through 129 )11 X-RAY DIFFRACTION 11 chain 'B' and (resid 38 through 50 )12 X-RAY DIFFRACTION 12 chain 'B' and (resid 51 through 66 )13 X-RAY DIFFRACTION 13 chain 'B' and (resid 67 through 78 )14 X-RAY DIFFRACTION 14 chain 'B' and (resid 79 through 92 )15 X-RAY DIFFRACTION 15 chain 'B' and (resid 93 through 102 )16 X-RAY DIFFRACTION 16 chain 'B
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  SAD / Resolution: 1.63 Å
SAD / Resolution: 1.63 Å  Authors
Authors Citation
Citation Journal: To be Published
Journal: To be Published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4hci.cif.gz
4hci.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4hci.ent.gz
pdb4hci.ent.gz PDB format
PDB format 4hci.json.gz
4hci.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/hc/4hci
https://data.pdbj.org/pub/pdb/validation_reports/hc/4hci ftp://data.pdbj.org/pub/pdb/validation_reports/hc/4hci
ftp://data.pdbj.org/pub/pdb/validation_reports/hc/4hci Links
Links Assembly
Assembly


 Components
Components

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 19-BM / Wavelength: 0.97926 Å
/ Beamline: 19-BM / Wavelength: 0.97926 Å Processing
Processing SAD / Resolution: 1.63→24.521 Å / SU ML: 0.15 / Isotropic thermal model: mixed / Cross valid method: THROUGHOUT / σ(F): 0 / Phase error: 26.49 / Stereochemistry target values: ML
SAD / Resolution: 1.63→24.521 Å / SU ML: 0.15 / Isotropic thermal model: mixed / Cross valid method: THROUGHOUT / σ(F): 0 / Phase error: 26.49 / Stereochemistry target values: ML Movie
Movie Controller
Controller




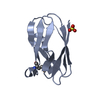




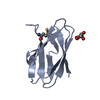


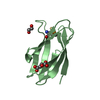

 PDBj
PDBj

