+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3zke | ||||||
|---|---|---|---|---|---|---|---|
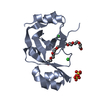
| Title | Structure of LC8 in complex with Nek9 peptide | ||||||
 Components Components |
| ||||||
 Keywords Keywords | CONTRACTILE PROTEIN/PEPTIDE / CONTRACTILE PROTEIN-PEPTIDE COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationnitric-oxide synthase inhibitor activity / deoxyribonuclease inhibitor activity / negative regulation of DNA strand resection involved in replication fork processing / negative regulation of phosphorylation / intraciliary retrograde transport / Activation of BIM and translocation to mitochondria / motile cilium assembly / Activation of NIMA Kinases NEK9, NEK6, NEK7 / ciliary tip / Intraflagellar transport ...nitric-oxide synthase inhibitor activity / deoxyribonuclease inhibitor activity / negative regulation of DNA strand resection involved in replication fork processing / negative regulation of phosphorylation / intraciliary retrograde transport / Activation of BIM and translocation to mitochondria / motile cilium assembly / Activation of NIMA Kinases NEK9, NEK6, NEK7 / ciliary tip / Intraflagellar transport / Nuclear Pore Complex (NPC) Disassembly / negative regulation of nitric oxide biosynthetic process / dynein complex / COPI-independent Golgi-to-ER retrograde traffic / cytoplasmic dynein complex / Macroautophagy / dynein intermediate chain binding / tertiary granule membrane / ficolin-1-rich granule membrane / protein kinase activator activity / spermatid development / enzyme inhibitor activity / positive regulation of insulin secretion involved in cellular response to glucose stimulus / COPI-mediated anterograde transport / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / axon cytoplasm / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / substantia nigra development / Recruitment of mitotic centrosome proteins and complexes / MHC class II antigen presentation / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / regulation of mitotic cell cycle / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / Resolution of Sister Chromatid Cohesion / AURKA Activation by TPX2 / RHO GTPases Activate Formins / kinetochore / HCMV Early Events / Aggrephagy / mitotic spindle / Separation of Sister Chromatids / Regulation of PLK1 Activity at G2/M Transition / mitotic cell cycle / site of double-strand break / scaffold protein binding / microtubule / cytoskeleton / non-specific serine/threonine protein kinase / cilium / cell division / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / DNA damage response / centrosome / Neutrophil degranulation / protein kinase binding / protein-containing complex binding / enzyme binding / mitochondrion / ATP binding / metal ion binding / identical protein binding / nucleus / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Gallego, P. / Velazquez-Campoy, A. / Regue, L. / Roig, J. / Reverter, D. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2013 Journal: J.Biol.Chem. / Year: 2013Title: Structural Analysis of the Regulation of the Dynll/Lc8 Binding to Nek9 by Phosphorylation Authors: Gallego, P. / Velazquez-Campoy, A. / Regue, L. / Roig, J. / Reverter, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3zke.cif.gz 3zke.cif.gz | 125.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3zke.ent.gz pdb3zke.ent.gz | 100.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3zke.json.gz 3zke.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  3zke_validation.pdf.gz 3zke_validation.pdf.gz | 499.6 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  3zke_full_validation.pdf.gz 3zke_full_validation.pdf.gz | 509.2 KB | Display | |
| Data in XML |  3zke_validation.xml.gz 3zke_validation.xml.gz | 23.1 KB | Display | |
| Data in CIF |  3zke_validation.cif.gz 3zke_validation.cif.gz | 33.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zk/3zke https://data.pdbj.org/pub/pdb/validation_reports/zk/3zke ftp://data.pdbj.org/pub/pdb/validation_reports/zk/3zke ftp://data.pdbj.org/pub/pdb/validation_reports/zk/3zke | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS ensembles :
NCS oper:
|
- Components
Components
| #1: Protein | Mass: 10381.899 Da / Num. of mol.: 6 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Production host: HOMO SAPIENS (human) / Production host:  #2: Protein/peptide | Mass: 1118.264 Da / Num. of mol.: 6 / Source method: obtained synthetically / Source: (synth.)  HOMO SAPIENS (human) / References: UniProt: Q6PKF2, UniProt: Q8TD19*PLUS HOMO SAPIENS (human) / References: UniProt: Q6PKF2, UniProt: Q8TD19*PLUS#3: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.04 Å3/Da / Density % sol: 39.68 % / Description: NONE |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALBA ALBA  / Beamline: XALOC / Wavelength: 0.979494 / Beamline: XALOC / Wavelength: 0.979494 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.979494 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→44.63 Å / Num. obs: 28774 / % possible obs: 97.6 % / Observed criterion σ(I): 2 / Redundancy: 3.2 % / Rmerge(I) obs: 0.08 / Net I/σ(I): 8 |
| Reflection shell | Resolution: 2.2→2.32 Å / Redundancy: 3.1 % / Rmerge(I) obs: 0.53 / Mean I/σ(I) obs: 2.7 / % possible all: 95.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.2→82.69 Å / Cor.coef. Fo:Fc: 0.952 / Cor.coef. Fo:Fc free: 0.938 / SU B: 5.483 / SU ML: 0.14 / Cross valid method: THROUGHOUT / ESU R: 0.332 / ESU R Free: 0.214 / Stereochemistry target values: MAXIMUM LIKELIHOOD MOLECULAR REPLACEMENT / Resolution: 2.2→82.69 Å / Cor.coef. Fo:Fc: 0.952 / Cor.coef. Fo:Fc free: 0.938 / SU B: 5.483 / SU ML: 0.14 / Cross valid method: THROUGHOUT / ESU R: 0.332 / ESU R Free: 0.214 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. RESIDUES 1-5 DISORDERED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 38.801 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→82.69 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj

















