[English] 日本語
 Yorodumi
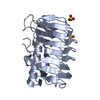
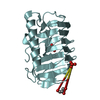
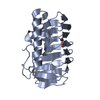
Yorodumi- PDB-2xqq: Human dynein light chain (DYNLL2) in complex with an in vitro evo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2xqq | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human dynein light chain (DYNLL2) in complex with an in vitro evolved peptide (Ac-SRGTQTE). | ||||||
 Components Components |
| ||||||
 Keywords Keywords | PROTEIN TRANSPORT / DIMER INTERFACE | ||||||
| Function / homology |  Function and homology information Function and homology informationmyosin V complex / Activation of BMF and translocation to mitochondria / 9+0 non-motile cilium / ciliary tip / Intraflagellar transport / COPI-independent Golgi-to-ER retrograde traffic / cytoplasmic dynein complex / Macroautophagy / dynein intermediate chain binding / microtubule-based process ...myosin V complex / Activation of BMF and translocation to mitochondria / 9+0 non-motile cilium / ciliary tip / Intraflagellar transport / COPI-independent Golgi-to-ER retrograde traffic / cytoplasmic dynein complex / Macroautophagy / dynein intermediate chain binding / microtubule-based process / COPI-mediated anterograde transport / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / MHC class II antigen presentation / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / Resolution of Sister Chromatid Cohesion / RHO GTPases Activate Formins / HCMV Early Events / Aggrephagy / Separation of Sister Chromatids / scaffold protein binding / microtubule / cytoskeleton / postsynapse / postsynaptic density / cilium / centrosome / protein-containing complex binding / glutamatergic synapse / identical protein binding / nucleus / membrane / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human)SYNTHETIC CONSTRUCT (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.31 Å MOLECULAR REPLACEMENT / Resolution: 1.31 Å | ||||||
 Authors Authors | Rapali, P. / Radnai, L. / Suveges, D. / Hetenyi, C. / Harmat, V. / Tolgyesi, F. / Wahlgren, W.Y. / Katona, G. / Nyitray, L. / Pal, G. | ||||||
 Citation Citation |  Journal: Plos One / Year: 2011 Journal: Plos One / Year: 2011Title: Directed Evolution Reveals the Binding Motif Preference of the Lc8/Dynll Hub Protein and Predicts Large Numbers of Novel Binders in the Human Proteome Authors: Rapali, P. / Radnai, L. / Suveges, D. / Harmat, V. / Tolgyesi, F. / Wahlgren, W.Y. / Katona, G. / Nyitray, L. / Pal, G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2xqq.cif.gz 2xqq.cif.gz | 183 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2xqq.ent.gz pdb2xqq.ent.gz | 147.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2xqq.json.gz 2xqq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xq/2xqq https://data.pdbj.org/pub/pdb/validation_reports/xq/2xqq ftp://data.pdbj.org/pub/pdb/validation_reports/xq/2xqq ftp://data.pdbj.org/pub/pdb/validation_reports/xq/2xqq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3p8mC  1cmiS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||
| 2 | 
| ||||||||||||||||
| 3 | 
| ||||||||||||||||
| Unit cell |
| ||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
| #1: Protein | Mass: 10364.847 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PET-15B / Production host: HOMO SAPIENS (human) / Plasmid: PET-15B / Production host:  #2: Protein/peptide | Mass: 820.825 Da / Num. of mol.: 4 / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) #3: Chemical | ChemComp-ACT / | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.7 Å3/Da / Density % sol: 27.2 % / Description: NONE |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 4.6 Details: HANGING DROP VAPOR DIFFUSION AT 293K WITH A RESERVOIR SOLUTION 31% PEG 4000, 0.4 M NH4AC, 0.1 M NAAC PH 4.6, 2UL RESERVOIR SOLUTION AND 2UL PROTEIN SOLUTION |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.93 / Beamline: ID29 / Wavelength: 0.93 |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Nov 3, 2009 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.93 Å / Relative weight: 1 |
| Reflection | Resolution: 1.31→151.84 Å / Num. obs: 81872 / % possible obs: 96 % / Observed criterion σ(I): 2 / Redundancy: 7.6 % / Biso Wilson estimate: 10.5 Å2 / Rmerge(I) obs: 0.07 / Net I/σ(I): 15.66 |
| Reflection shell | Resolution: 1.31→1.34 Å / Redundancy: 3.53 % / Rmerge(I) obs: 0.48 / Mean I/σ(I) obs: 2.93 / % possible all: 67.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1CMI Resolution: 1.31→151.84 Å / Cor.coef. Fo:Fc: 0.976 / Cor.coef. Fo:Fc free: 0.966 / SU B: 1.17 / SU ML: 0.023 / Cross valid method: THROUGHOUT / ESU R: 0.047 / ESU R Free: 0.045 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 10.471 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.31→151.84 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller









 PDBj
PDBj



















