[English] 日本語
 Yorodumi
Yorodumi- PDB-3zda: Structure of E. coli ExoIX in complex with a fragment of the Flap... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3zda | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of E. coli ExoIX in complex with a fragment of the Flap1 DNA oligonucleotide, potassium and magnesium | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/DNA / HYDROLASE-DNA COMPLEX / FLAP ENDONUCLEASE / DNA BINDING | ||||||
| Function / homology |  Function and homology information Function and homology informationDNA replication, Okazaki fragment processing / 5'-flap endonuclease activity / 5'-3' exonuclease activity / potassium ion binding / Hydrolases; Acting on ester bonds / magnesium ion binding / DNA binding Similarity search - Function | ||||||
| Biological species |  SYNTHETIC CONSTRUCT (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | ||||||
 Authors Authors | Hemsworth, G.R. / Anstey-Gilbert, C.S. / Flemming, C.S. / Hodskinson, M.R.G. / Zhang, J. / Sedelnikova, S.E. / Stillman, T.J. / Sayers, J.R. / Artymiuk, P.J. | ||||||
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2013 Journal: Nucleic Acids Res. / Year: 2013Title: The Structure of E. Coli Exoix - Implications for DNA Binding and Catalysis in Flap Endonucleases Authors: Anstey-Gilbert, C.S. / Hemsworth, G.R. / Flemming, C.S. / Hodskinson, M.R.G. / Zhang, J. / Sedelnikova, S.E. / Stillman, T.J. / Sayers, J.R. / Artymiuk, P.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3zda.cif.gz 3zda.cif.gz | 78.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3zda.ent.gz pdb3zda.ent.gz | 54.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3zda.json.gz 3zda.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zd/3zda https://data.pdbj.org/pub/pdb/validation_reports/zd/3zda ftp://data.pdbj.org/pub/pdb/validation_reports/zd/3zda ftp://data.pdbj.org/pub/pdb/validation_reports/zd/3zda | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3zd8C  3zd9SC  3zdbC  3zdcC  3zddC  3zdeC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 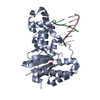
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 28203.158 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: Q8X6R9, Hydrolases; Acting on ester bonds |
|---|
-DNA chain , 2 types, 2 molecules BC
| #2: DNA chain | Mass: 1191.818 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) |
|---|---|
| #3: DNA chain | Mass: 1818.231 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) |
-Non-polymers , 5 types, 247 molecules 







| #4: Chemical | ChemComp-K / |
|---|---|
| #5: Chemical | ChemComp-MG / |
| #6: Chemical | ChemComp-PIV / |
| #7: Chemical | ChemComp-PO4 / |
| #8: Water | ChemComp-HOH / |
-Details
| Sequence details | FRAGMENT OF LARGER DNA BOUND TO PROTEIN. SEQUENCE IS ACTUALLY UNKNOWN, CRYSTAL LIKELY HAS MIXED ...FRAGMENT OF LARGER DNA BOUND TO PROTEIN. SEQUENCE IS ACTUALLY UNKNOWN, CRYSTAL LIKELY HAS MIXED DNAS BOUND HERE. |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 47 % / Description: NONE |
|---|---|
| Crystal grow | pH: 6 Details: 0.2 M MAGNESIUM ACETATE, 15% (W/V) PEG-3350, pH 6.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.976 / Beamline: I03 / Wavelength: 0.976 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Dec 16, 2008 / Details: MIRRORS |
| Radiation | Monochromator: DOUBLE CRYSTAL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.976 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→51.64 Å / Num. obs: 57905 / % possible obs: 99.9 % / Observed criterion σ(I): 6 / Redundancy: 9.9 % / Rmerge(I) obs: 0.1 / Net I/σ(I): 22.5 |
| Reflection shell | Resolution: 1.5→1.58 Å / Redundancy: 7 % / Rmerge(I) obs: 0.48 / Mean I/σ(I) obs: 3.4 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3ZD9 Resolution: 1.5→51.64 Å / Cor.coef. Fo:Fc: 0.954 / Cor.coef. Fo:Fc free: 0.941 / SU B: 1.333 / SU ML: 0.051 / Cross valid method: THROUGHOUT / ESU R: 0.074 / ESU R Free: 0.075 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 25.205 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→51.64 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj




