Entry Database : PDB / ID : 3wp5Title The crystal structure of mutant CDBFV E109A from Neocallimastix patriciarum CDBFV Keywords / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Neocallimastix patriciarum (fungus)Method / / / Resolution : 1.32 Å Authors Cheng, Y.S. / Chen, C.C. / Huang, C.H. / Huang, T.Y. / Ko, T.P. / Huang, J.W. / Wu, T.H. / Liu, J.R. / Guo, R.T. Journal : J.Biol.Chem. / Year : 2014Title : Structural analysis of a glycoside hydrolase family 11 xylanase from Neocallimastix patriciarum: insights into the molecular basis of a thermophilic enzyme.Authors : Cheng, Y.S. / Chen, C.C. / Huang, C.H. / Ko, T.P. / Luo, W. / Huang, J.W. / Liu, J.R. / Guo, R.T. History Deposition Jan 9, 2014 Deposition site / Processing site Revision 1.0 Mar 19, 2014 Provider / Type Revision 1.1 Dec 13, 2017 Group / Category / Item Revision 1.2 Dec 25, 2019 Group / Category Item _citation.journal_volume / _citation.page_first ... _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_PubMed / _citation.title Revision 1.3 Nov 8, 2023 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accessionRevision 1.4 Nov 13, 2024 Group / Category / pdbx_modification_feature / Item
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Neocallimastix patriciarum (fungus)
Neocallimastix patriciarum (fungus) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.32 Å
MOLECULAR REPLACEMENT / Resolution: 1.32 Å  Authors
Authors Citation
Citation Journal: J.Biol.Chem. / Year: 2014
Journal: J.Biol.Chem. / Year: 2014 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3wp5.cif.gz
3wp5.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3wp5.ent.gz
pdb3wp5.ent.gz PDB format
PDB format 3wp5.json.gz
3wp5.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/wp/3wp5
https://data.pdbj.org/pub/pdb/validation_reports/wp/3wp5 ftp://data.pdbj.org/pub/pdb/validation_reports/wp/3wp5
ftp://data.pdbj.org/pub/pdb/validation_reports/wp/3wp5

 Links
Links Assembly
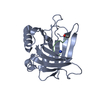
Assembly
 Components
Components Neocallimastix patriciarum (fungus) / Plasmid: pPICZalphaA / Production host:
Neocallimastix patriciarum (fungus) / Plasmid: pPICZalphaA / Production host:  Komagataella pastoris (fungus) / References: UniProt: Q9UV68*PLUS
Komagataella pastoris (fungus) / References: UniProt: Q9UV68*PLUS X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  NSRRC
NSRRC  / Beamline: BL13B1 / Wavelength: 1 Å
/ Beamline: BL13B1 / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller











 PDBj
PDBj
