[English] 日本語
 Yorodumi
Yorodumi- PDB-3w0j: Crystal Structure of Rat VDR Ligand Binding Domain in Complex wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3w0j | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Rat VDR Ligand Binding Domain in Complex with Novel Nonsecosteroidal Ligands | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION / GENE REGULATION | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of bone trabecula formation / Vitamin D (calciferol) metabolism / enucleate erythrocyte development / positive regulation of type II interferon-mediated signaling pathway / regulation of RNA biosynthetic process / androgen biosynthetic process / positive regulation of G0 to G1 transition / SUMOylation of intracellular receptors / retinal pigment epithelium development / Nuclear Receptor transcription pathway ...negative regulation of bone trabecula formation / Vitamin D (calciferol) metabolism / enucleate erythrocyte development / positive regulation of type II interferon-mediated signaling pathway / regulation of RNA biosynthetic process / androgen biosynthetic process / positive regulation of G0 to G1 transition / SUMOylation of intracellular receptors / retinal pigment epithelium development / Nuclear Receptor transcription pathway / G0 to G1 transition / thyroid hormone receptor signaling pathway / response to bile acid / mammary gland branching involved in thelarche / dense fibrillar component / core mediator complex / positive regulation of parathyroid hormone secretion / regulation of vitamin D receptor signaling pathway / apoptotic process involved in mammary gland involution / positive regulation of apoptotic process involved in mammary gland involution / vitamin D binding / calcitriol binding / cellular response to vitamin D / lithocholic acid binding / nuclear retinoic acid receptor binding / nuclear receptor-mediated bile acid signaling pathway / bile acid nuclear receptor activity / positive regulation of hepatocyte proliferation / ventricular trabecula myocardium morphogenesis / mediator complex / positive regulation of keratinocyte differentiation / thyroid hormone generation / Generic Transcription Pathway / response to aldosterone / peroxisome proliferator activated receptor binding / embryonic heart tube development / cellular response to thyroid hormone stimulus / phosphate ion transmembrane transport / vitamin D receptor signaling pathway / positive regulation of vitamin D receptor signaling pathway / nuclear vitamin D receptor binding / negative regulation of ossification / embryonic hindlimb morphogenesis / nuclear thyroid hormone receptor binding / lens development in camera-type eye / intestinal absorption / embryonic hemopoiesis / megakaryocyte development / cellular response to hepatocyte growth factor stimulus / cellular response to steroid hormone stimulus / positive regulation of intracellular estrogen receptor signaling pathway / negative regulation of neuron differentiation / epithelial cell proliferation involved in mammary gland duct elongation / histone acetyltransferase binding / LBD domain binding / erythrocyte development / RSV-host interactions / fat cell differentiation / mammary gland branching involved in pregnancy / decidualization / nuclear steroid receptor activity / regulation of calcium ion transport / monocyte differentiation / general transcription initiation factor binding / animal organ regeneration / negative regulation of keratinocyte proliferation / hematopoietic stem cell differentiation / positive regulation of transcription initiation by RNA polymerase II / ubiquitin ligase complex / nuclear receptor-mediated steroid hormone signaling pathway / embryonic placenta development / nuclear retinoid X receptor binding / heterochromatin / RNA polymerase II preinitiation complex assembly / retinoic acid receptor signaling pathway / keratinocyte differentiation / intracellular receptor signaling pathway / lactation / : / Regulation of lipid metabolism by PPARalpha / peroxisome proliferator activated receptor signaling pathway / T-tubule / BMAL1:CLOCK,NPAS2 activates circadian expression / Activation of gene expression by SREBF (SREBP) / positive regulation of erythrocyte differentiation / cellular response to epidermal growth factor stimulus / animal organ morphogenesis / nuclear estrogen receptor binding / nuclear receptor binding / skeletal system development / transcription coregulator activity / apoptotic signaling pathway / promoter-specific chromatin binding / positive regulation of transcription elongation by RNA polymerase II / liver development / mRNA transcription by RNA polymerase II / Heme signaling / euchromatin / PPARA activates gene expression / Transcriptional activation of mitochondrial biogenesis Similarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.84 Å MOLECULAR REPLACEMENT / Resolution: 1.84 Å | ||||||
 Authors Authors | Shimizu, T. / Asano, L. / Kuwabara, N. / Ito, I. / Waku, T. / Yanagisawa, J. / Miyachi, H. | ||||||
 Citation Citation |  Journal: Febs Lett. / Year: 2013 Journal: Febs Lett. / Year: 2013Title: Structural basis for vitamin D receptor agonism by novel non-secosteroidal ligands. Authors: Asano, L. / Ito, I. / Kuwabara, N. / Waku, T. / Yanagisawa, J. / Miyachi, H. / Shimizu, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3w0j.cif.gz 3w0j.cif.gz | 68.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3w0j.ent.gz pdb3w0j.ent.gz | 49.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3w0j.json.gz 3w0j.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/w0/3w0j https://data.pdbj.org/pub/pdb/validation_reports/w0/3w0j ftp://data.pdbj.org/pub/pdb/validation_reports/w0/3w0j ftp://data.pdbj.org/pub/pdb/validation_reports/w0/3w0j | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3w0gC  3w0hC  3w0iC  1rk3S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 28755.025 Da / Num. of mol.: 1 Fragment: LIGAND BINDING DOMAIN, UNP residues 121-164, 212-420 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Protein/peptide | Mass: 1570.898 Da / Num. of mol.: 1 / Fragment: DRIP 205 NR2 BOX PEPTIDE, UNP residues 640-652 / Source method: obtained synthetically / Details: This sequence occurs naturally in humans. / Source: (synth.)  Homo sapiens (human) / References: UniProt: Q15648 Homo sapiens (human) / References: UniProt: Q15648 |
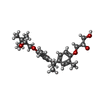
| #3: Chemical | ChemComp-T08 / ( |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.26 Å3/Da / Density % sol: 45.64 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7 Details: 0.1M MOPS (pH7.0), 16-18% PEG 4000, 0.2M sodium formate, 5% ethylene glycol , VAPOR DIFFUSION, HANGING DROP, temperature 293.0K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL32XU / Wavelength: 1 Å / Beamline: BL32XU / Wavelength: 1 Å |
| Detector | Type: RAYONIX MX225HE / Detector: CCD / Date: May 21, 2011 |
| Radiation | Monochromator: Si 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→40.86 Å / Num. all: 17613 / Num. obs: 17224 / % possible obs: 97.79 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 |
| Reflection shell | Resolution: 1.8→1.86 Å / % possible all: 97.79 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1RK3 Resolution: 1.84→40.86 Å / Cor.coef. Fo:Fc: 0.944 / Cor.coef. Fo:Fc free: 0.93 / SU B: 5.221 / SU ML: 0.144 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.231 / ESU R Free: 0.188 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 38.191 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.84→40.86 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2→2.052 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller











 PDBj
PDBj