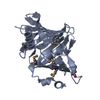
Entry Database : PDB / ID : 3posTitle Crystal structure of the globular domain of human calreticulin Calreticulin Keywords / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 1.65 Å Authors Chouquet, A. / Paidassi, H. / Ling, W.-L. / Frachet, P. / Houen, G. / Arlaud, G.J. / Gaboriaud, C. Journal : Plos One / Year : 2011Title : X-ray structure of the human calreticulin globular domain reveals a Peptide-binding area and suggests a multi-molecular mechanismAuthors : Chouquet, A. / Paidassi, H. / Ling, W.L. / Frachet, P. / Houen, G. / Arlaud, G.J. / Gaboriaud, C. History Deposition Nov 23, 2010 Deposition site / Processing site Revision 1.0 Mar 9, 2011 Provider / Type Revision 1.1 Jul 13, 2011 Group Revision 1.2 Aug 9, 2017 Group / Refinement description / Source and taxonomyCategory / entity_src_gen / software / Item Revision 1.3 Oct 16, 2024 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Structure summary Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature / pdbx_struct_conn_angle / struct_conn / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.65 Å
MOLECULAR REPLACEMENT / Resolution: 1.65 Å  Authors
Authors Citation
Citation Journal: Plos One / Year: 2011
Journal: Plos One / Year: 2011 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3pos.cif.gz
3pos.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3pos.ent.gz
pdb3pos.ent.gz PDB format
PDB format 3pos.json.gz
3pos.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/po/3pos
https://data.pdbj.org/pub/pdb/validation_reports/po/3pos ftp://data.pdbj.org/pub/pdb/validation_reports/po/3pos
ftp://data.pdbj.org/pub/pdb/validation_reports/po/3pos Links
Links Assembly
Assembly



 Components
Components Homo sapiens (human) / Gene: CALR, CRTC / Plasmid: pHFX-CRT / Production host:
Homo sapiens (human) / Gene: CALR, CRTC / Plasmid: pHFX-CRT / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID29 / Wavelength: 0.97925 Å
/ Beamline: ID29 / Wavelength: 0.97925 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 1.65→19.94 Å / Cor.coef. Fo:Fc: 0.9545 / Cor.coef. Fo:Fc free: 0.9386 / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
MOLECULAR REPLACEMENT / Resolution: 1.65→19.94 Å / Cor.coef. Fo:Fc: 0.9545 / Cor.coef. Fo:Fc free: 0.9386 / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber Movie
Movie Controller
Controller













 PDBj
PDBj









