[English] 日本語
 Yorodumi
Yorodumi- PDB-3oyw: Crystal structure of human galectin-1 in complex with thiodigalac... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3oyw | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
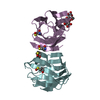
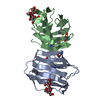
| Title | Crystal structure of human galectin-1 in complex with thiodigalactoside | ||||||||||||
 Components Components | (Galectin-1) x 2 | ||||||||||||
 Keywords Keywords | CARBOHYDRATE BINDING PROTEIN / Galectin / Lactobionic acid | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationgalectin complex / lactose binding / negative regulation of T-helper 17 cell lineage commitment / myoblast differentiation / plasma cell differentiation / T cell costimulation / laminin binding / Post-translational protein phosphorylation / : / cell-cell adhesion ...galectin complex / lactose binding / negative regulation of T-helper 17 cell lineage commitment / myoblast differentiation / plasma cell differentiation / T cell costimulation / laminin binding / Post-translational protein phosphorylation / : / cell-cell adhesion / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / positive regulation of inflammatory response / regulation of apoptotic process / positive regulation of viral entry into host cell / positive regulation of canonical NF-kappaB signal transduction / positive regulation of apoptotic process / endoplasmic reticulum lumen / receptor ligand activity / apoptotic process / extracellular space / RNA binding / extracellular exosome / extracellular region / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FOURIER SYNTHESIS / Resolution: 2.5 Å FOURIER SYNTHESIS / Resolution: 2.5 Å | ||||||||||||
 Authors Authors | Blanchard, H. / Collins, P.M. | ||||||||||||
 Citation Citation |  Journal: CANCER LETT. / Year: 2010 Journal: CANCER LETT. / Year: 2010Title: Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model Authors: Stannard, K.A. / Collins, P.M. / Ito, K. / Sullivan, E.M. / Scott, S.A. / Gabutero, E. / Darren Grice, I. / Low, P. / Nilsson, U.J. / Leffler, H. / Blanchard, H. / Ralph, S.J. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3oyw.cif.gz 3oyw.cif.gz | 68.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3oyw.ent.gz pdb3oyw.ent.gz | 50.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3oyw.json.gz 3oyw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oy/3oyw https://data.pdbj.org/pub/pdb/validation_reports/oy/3oyw ftp://data.pdbj.org/pub/pdb/validation_reports/oy/3oyw ftp://data.pdbj.org/pub/pdb/validation_reports/oy/3oyw | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 14707.594 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: LGALS1 / Production host: Homo sapiens (human) / Gene: LGALS1 / Production host:  | ||||
|---|---|---|---|---|---|
| #2: Protein | Mass: 14767.713 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: LGALS1 / Production host: Homo sapiens (human) / Gene: LGALS1 / Production host:  | ||||
| #3: Polysaccharide |   Type: oligosaccharide, Oligosaccharide / Class: Substrate analog / Mass: 358.362 Da / Num. of mol.: 2 Type: oligosaccharide, Oligosaccharide / Class: Substrate analog / Mass: 358.362 Da / Num. of mol.: 2Source method: isolated from a genetically manipulated source Details: oligosaccharide with S-glycosidic bond between monosaccharides, and with reducing-end-to-reducing-end glycosidic bond References: thiodigalactoside #4: Water | ChemComp-HOH / | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.46 Å3/Da / Density % sol: 49.91 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 6.2 Details: 4-8 microlitre drops consisting of equal volumes of protein solution (20 mM sodium potassium phosphate buffer, pH 7.0, and protein at concentration of 10 mg/mL) and reservoir solution (0.2 M ...Details: 4-8 microlitre drops consisting of equal volumes of protein solution (20 mM sodium potassium phosphate buffer, pH 7.0, and protein at concentration of 10 mg/mL) and reservoir solution (0.2 M ammonium sulphate, 25% w/v polyethylene glycol 4000, 0.1M sodium acetate trihydrate, pH 6.2), VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: MACSCIENCE / Wavelength: 1.5418 Å ROTATING ANODE / Type: MACSCIENCE / Wavelength: 1.5418 Å |
| Detector | Type: BRUKER SMART 6000 / Detector: CCD / Date: May 9, 2008 |
| Radiation | Monochromator: osmic mirrors / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→55.9 Å / Num. all: 10154 / Num. obs: 10154 / % possible obs: 95.2 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 |
| Reflection shell | Resolution: 2.5→2.6 Å / % possible all: 80.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS / Resolution: 2.5→55.9 Å / Cor.coef. Fo:Fc: 0.956 / Cor.coef. Fo:Fc free: 0.908 / SU B: 8.532 / SU ML: 0.194 / Cross valid method: THROUGHOUT / ESU R: 0.698 / ESU R Free: 0.302 / Stereochemistry target values: MAXIMUM LIKELIHOOD FOURIER SYNTHESIS / Resolution: 2.5→55.9 Å / Cor.coef. Fo:Fc: 0.956 / Cor.coef. Fo:Fc free: 0.908 / SU B: 8.532 / SU ML: 0.194 / Cross valid method: THROUGHOUT / ESU R: 0.698 / ESU R Free: 0.302 / Stereochemistry target values: MAXIMUM LIKELIHOODDetails: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES: REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27.616 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→55.9 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.495→2.56 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller


















 PDBj
PDBj
