[English] 日本語
 Yorodumi
Yorodumi- PDB-3oyl: Crystal structure of the PFV S217H mutant intasome bound to magne... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3oyl | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the PFV S217H mutant intasome bound to magnesium and the INSTI MK2048 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | RECOMBINATION / VIRAL PROTEIN/DNA / PROTEIN-DNA COMPLEX / TETRAMER / DNA INTEGRATION / ENDONUCLEASE / METAL-BINDING / MULTIFUNCTIONAL ENZYME / NUCLEASE / NUCLEOTIDYLTRANSFERASE / NUCLEUS / TRANSFERASE / VIRAL NUCLEOPROTEIN / VIRION / DNA-BINDING / ZINC BINDING / HHCC MOTIF / VIRAL PROTEIN / INHIBITOR / DNA-BINDING PROTEIN-DNA complex / VIRAL PROTEIN-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationribonuclease H / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / DNA integration / virion component / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / telomerase activity / viral penetration into host nucleus / RNA-DNA hybrid ribonuclease activity ...ribonuclease H / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / DNA integration / virion component / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / telomerase activity / viral penetration into host nucleus / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / host cell cytoplasm / DNA-directed DNA polymerase activity / symbiont entry into host cell / host cell nucleus / proteolysis / RNA binding / metal ion binding Similarity search - Function | ||||||
| Biological species |  Human spumaretrovirus Human spumaretrovirus | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.54 Å molecular replacement / Resolution: 2.54 Å | ||||||
 Authors Authors | Hare, S. / Cherepanov, P. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2010 Journal: Proc.Natl.Acad.Sci.USA / Year: 2010Title: Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Authors: Hare, S. / Vos, A.M. / Clayton, R.F. / Thuring, J.W. / Cummings, M.D. / Cherepanov, P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3oyl.cif.gz 3oyl.cif.gz | 156.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3oyl.ent.gz pdb3oyl.ent.gz | 116.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3oyl.json.gz 3oyl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oy/3oyl https://data.pdbj.org/pub/pdb/validation_reports/oy/3oyl ftp://data.pdbj.org/pub/pdb/validation_reports/oy/3oyl ftp://data.pdbj.org/pub/pdb/validation_reports/oy/3oyl | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3oyaC  3oybSC  3oycC  3oydC  3oyeC  3oyfC  3oygC  3oyhC  3oyiC  3oyjC  3oykC  3oymC  3oynC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 44507.762 Da / Num. of mol.: 2 / Fragment: UNP residues 752 to 1143 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human spumaretrovirus / Strain: HSRV2 / Gene: POL / Plasmid: pSSH6P-PFV-INFL / Production host: Human spumaretrovirus / Strain: HSRV2 / Gene: POL / Plasmid: pSSH6P-PFV-INFL / Production host:  |
|---|
-DNA chain , 2 types, 2 molecules CD
| #2: DNA chain | Mass: 5834.794 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: Donor DNA transferred strand |
|---|---|
| #3: DNA chain | Mass: 5195.399 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: Donor DNA transferred strand |
-Non-polymers , 7 types, 241 molecules 




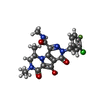







| #4: Chemical | ChemComp-ZN / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #5: Chemical | | #6: Chemical | ChemComp-GOL / #7: Chemical | ChemComp-NH4 / | #8: Chemical | #9: Chemical | ChemComp-ZZX / ( | #10: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.94 Å3/Da / Density % sol: 68.75 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 1.35 M ammonium sulfate, 25% (v/v) glycerol, 4.8% (v/v) 1,6-hexanediol, 50 mM Mes-NaOH, 1mM EDTA, pH 6.5, vapor diffusion, hanging drop, temperature 291K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.98011 Å / Beamline: PROXIMA 1 / Wavelength: 0.98011 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC / Detector: AREA DETECTOR / Date: Jun 8, 2010 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.98011 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.54→39.094 Å / Num. obs: 52924 / % possible obs: 99.7 % / Observed criterion σ(F): -3 / Observed criterion σ(I): -3 / Redundancy: 5.3 % / Rsym value: 0.093 / Net I/σ(I): 11.7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 3OYB Resolution: 2.54→39.09 Å / Cor.coef. Fo:Fc: 0.95 / Cor.coef. Fo:Fc free: 0.939 / WRfactor Rfree: 0.2224 / WRfactor Rwork: 0.2002 / Occupancy max: 1 / Occupancy min: 1 / FOM work R set: 0.846 / SU B: 7.179 / SU ML: 0.152 / SU R Cruickshank DPI: 0.2299 / SU Rfree: 0.1925 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.192 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 127.87 Å2 / Biso mean: 59.4226 Å2 / Biso min: 23.96 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.54→39.09 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.54→2.606 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller





























 PDBj
PDBj












































