[English] 日本語
 Yorodumi
Yorodumi- PDB-3hvt: STRUCTURAL BASIS OF ASYMMETRY IN THE HUMAN IMMUNODEFICIENCY VIRUS... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3hvt | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | STRUCTURAL BASIS OF ASYMMETRY IN THE HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 REVERSE TRANSCRIPTASE HETERODIMER | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | NUCLEOTIDYLTRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationHIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / host multivesicular body / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency ...HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / host multivesicular body / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / viral penetration into host nucleus / RNA stem-loop binding / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / lipid binding / symbiont entry into host cell / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.9 Å X-RAY DIFFRACTION / Resolution: 2.9 Å | |||||||||
 Authors Authors | Steitz, T.A. / Smerdon, S.J. / Jaeger, J. / Wang, J. / Kohlstaedt, L.A. / Chirino, A.J. / Friedman, J.M. / Rice, P.A. | |||||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.Usa / Year: 1994 Journal: Proc.Natl.Acad.Sci.Usa / Year: 1994Title: Structure of the binding site for nonnucleoside inhibitors of the reverse transcriptase of human immunodeficiency virus type 1. Authors: Smerdon, S.J. / Jager, J. / Wang, J. / Kohlstaedt, L.A. / Chirino, A.J. / Friedman, J.M. / Rice, P.A. / Steitz, T.A. #1:  Journal: To be Published Journal: To be PublishedTitle: Comparison of Three Different Crystal Forms Shows HIV-1 Reverse Transcriptase Displays an Internal Swivel Motion Authors: Jaeger, J. / Smerdon, S.J. / Wang, J. / Boisvert, D.C. / Steitz, T.A. #2:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1994 Journal: Proc.Natl.Acad.Sci.USA / Year: 1994Title: Structure of the Binding Site for Nonnucleoside Inhibitors of the Reverse Transcriptase of Human Immunodeficiency Virus Type 1 Authors: Smerdon, S.J. / Jaeger, J. / Wang, J. / Kohlstaedt, L.A. / Chirino, A.J. / Friedman, J. / Rice, P.A. / Steitz, T.A. #3:  Journal: Cold Spring Harbor Symp.Quant.Biol. / Year: 1993 Journal: Cold Spring Harbor Symp.Quant.Biol. / Year: 1993Title: Two DNA Polymerases: HIV Reverse Transcriptase and Klenow Fragment of E. Coli DNA Polymerase I. (In: DNA & Chromosomes: Abstracts of Papers Presented at the LVIII Cold Spring Harbor Symposium ...Title: Two DNA Polymerases: HIV Reverse Transcriptase and Klenow Fragment of E. Coli DNA Polymerase I. (In: DNA & Chromosomes: Abstracts of Papers Presented at the LVIII Cold Spring Harbor Symposium on Quantitative Biology) Authors: Steitz, T.A. / Smerdon, S.J. / Jaeger, J. / Wang, J. / Kohlstaedt, L.A. / Friedman, J. / Beese, L. / Rice, P.A. #4:  Journal: Science / Year: 1992 Journal: Science / Year: 1992Title: Crystal Structure at 3.5 Angstroms Resolution of HIV-1 Reverse Transcriptase Complexed with an Inhibitor Authors: Kohlstaedt, L.A. / Wang, J. / Friedman, J.M. / Rice, P.A. / Steitz, T.A. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3hvt.cif.gz 3hvt.cif.gz | 196.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3hvt.ent.gz pdb3hvt.ent.gz | 152.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3hvt.json.gz 3hvt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  3hvt_validation.pdf.gz 3hvt_validation.pdf.gz | 481.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  3hvt_full_validation.pdf.gz 3hvt_full_validation.pdf.gz | 577.1 KB | Display | |
| Data in XML |  3hvt_validation.xml.gz 3hvt_validation.xml.gz | 32.1 KB | Display | |
| Data in CIF |  3hvt_validation.cif.gz 3hvt_validation.cif.gz | 46.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hv/3hvt https://data.pdbj.org/pub/pdb/validation_reports/hv/3hvt ftp://data.pdbj.org/pub/pdb/validation_reports/hv/3hvt ftp://data.pdbj.org/pub/pdb/validation_reports/hv/3hvt | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO B 4 / 2: CIS PROLINE - PRO B 294 / 3: CIS PROLINE - PRO B 420 |
- Components
Components
| #1: Protein | Mass: 64004.336 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Genus: Lentivirus / References: UniProt: P03366, RNA-directed DNA polymerase Human immunodeficiency virus 1 / Genus: Lentivirus / References: UniProt: P03366, RNA-directed DNA polymerase |
|---|---|
| #2: Protein | Mass: 50055.551 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Genus: Lentivirus / References: UniProt: P03366, RNA-directed DNA polymerase Human immunodeficiency virus 1 / Genus: Lentivirus / References: UniProt: P03366, RNA-directed DNA polymerase |
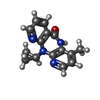
| #3: Chemical | ChemComp-NVP / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.46 Å3/Da / Density % sol: 64.5 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 7 / Method: vapor diffusion / Details: Kohlstaedt, L.A., (1992) Science, 256, 1783. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Reflection | *PLUS Highest resolution: 2.9 Å / Num. obs: 33169 / % possible obs: 0.96 % / Rmerge(I) obs: 0.09 |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.9→8 Å / σ(F): 2 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.9→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.266 / Rfactor Rwork: 0.266 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_d / Dev ideal: 2.5 |
 Movie
Movie Controller
Controller












 PDBj
PDBj