Entry Database : PDB / ID : 3ewtTitle Crystal Structure of calmodulin complexed with a peptide Calmodulin Tumor necrosis factor receptor superfamily member 6 Keywords / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)synthetic construct (others) Method / / Resolution : 2.4 Å Authors Jiang, T. / Cao, P. / Gong, Y. / Yu, H.J. / Gui, W.J. / Zhang, W.T. Journal : Acta Crystallogr.,Sect.D / Year : 2014Title : Structural insights into the mechanism of calmodulin binding to death receptors.Authors : Cao, P. / Zhang, W. / Gui, W. / Dong, Y. / Jiang, T. / Gong, Y. History Deposition Oct 16, 2008 Deposition site / Processing site Revision 1.0 Oct 20, 2009 Provider / Type Revision 1.1 Jul 13, 2011 Group Revision 1.2 Jun 18, 2014 Group Revision 1.3 Jan 1, 2020 Group / Source and taxonomyCategory citation / citation_author ... citation / citation_author / pdbx_entity_src_syn / struct_ref_seq_dif Item _citation.country / _citation.journal_id_CSD ... _citation.country / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.pdbx_database_id_PubMed / _citation.title / _citation_author.name / _pdbx_entity_src_syn.ncbi_taxonomy_id / _pdbx_entity_src_syn.organism_scientific / _struct_ref_seq_dif.details Revision 1.4 Nov 1, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å
MOLECULAR REPLACEMENT / Resolution: 2.4 Å  Authors
Authors Citation
Citation Journal: Acta Crystallogr.,Sect.D / Year: 2014
Journal: Acta Crystallogr.,Sect.D / Year: 2014 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3ewt.cif.gz
3ewt.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3ewt.ent.gz
pdb3ewt.ent.gz PDB format
PDB format 3ewt.json.gz
3ewt.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 3ewt_validation.pdf.gz
3ewt_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 3ewt_full_validation.pdf.gz
3ewt_full_validation.pdf.gz 3ewt_validation.xml.gz
3ewt_validation.xml.gz 3ewt_validation.cif.gz
3ewt_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/ew/3ewt
https://data.pdbj.org/pub/pdb/validation_reports/ew/3ewt ftp://data.pdbj.org/pub/pdb/validation_reports/ew/3ewt
ftp://data.pdbj.org/pub/pdb/validation_reports/ew/3ewt

 Links
Links Assembly
Assembly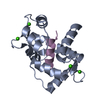
 Components
Components Homo sapiens (human) / Plasmid: pET28a / Production host:
Homo sapiens (human) / Plasmid: pET28a / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å
ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller












 PDBj
PDBj


































