[English] 日本語
 Yorodumi
Yorodumi- PDB-2ycz: TURKEY BETA1 ADRENERGIC RECEPTOR WITH STABILISING MUTATIONS AND B... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2ycz | ||||||
|---|---|---|---|---|---|---|---|
| Title | TURKEY BETA1 ADRENERGIC RECEPTOR WITH STABILISING MUTATIONS AND BOUND ANTAGONIST IODOCYANOPINDOLOL | ||||||
 Components Components | BETA-1 ADRENERGIC RECEPTOR | ||||||
 Keywords Keywords | RECEPTOR / GPCR / TRANSDUCER / ANTAGONIST BOUND FORM / INTEGRAL MEMBRANE PROTEIN / G-PROTEIN COUPLED RECEPTOR / THERMOSTABILISING POINT MUTATIONS / SEVEN-HELIX RECEPTOR / 7TM RECEPTOR | ||||||
| Function / homology |  Function and homology information Function and homology informationbeta1-adrenergic receptor activity / positive regulation of heart contraction / regulation of circadian sleep/wake cycle, sleep / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / adenylate cyclase-activating adrenergic receptor signaling pathway / early endosome / positive regulation of MAPK cascade / membrane / identical protein binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.65 Å MOLECULAR REPLACEMENT / Resolution: 3.65 Å | ||||||
 Authors Authors | Moukhametzianov, R. / Warne, T. / Edwards, P.C. / Serrano-Vega, M.J. / Leslie, A.G.W. / Tate, C.G. / Schertler, G.F.X. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2011 Journal: Proc.Natl.Acad.Sci.USA / Year: 2011Title: Two Distinct Conformations of Helix 6 Observed in Antagonist-Bound Structures of a {Beta}1- Adrenergic Receptor. Authors: Moukhametzianov, R. / Warne, T. / Edwards, P.C. / Serrano-Vega, M.J. / Leslie, A.G. / Tate, C.G. / Schertler, G.F. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2ycz.cif.gz 2ycz.cif.gz | 130.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2ycz.ent.gz pdb2ycz.ent.gz | 102.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2ycz.json.gz 2ycz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yc/2ycz https://data.pdbj.org/pub/pdb/validation_reports/yc/2ycz ftp://data.pdbj.org/pub/pdb/validation_reports/yc/2ycz ftp://data.pdbj.org/pub/pdb/validation_reports/yc/2ycz | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2ycwC  2ycxC  2ycyC  2vt4S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
|
- Components
Components
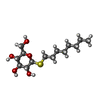
| #1: Protein | Mass: 35752.598 Da / Num. of mol.: 2 / Fragment: RESIDUES 33-243,272-276,279-367 / Mutation: YES Source method: isolated from a genetically manipulated source Details: RESIDUES 3-32 AT THE N-TERMINUS AND RESIDUES 244-271 AND 277-278 OF THE THIRD INTRACELLULAR LOOP WERE DELETED FROM THE CONSTRUCT. THE CONSTRUCT WAS TRUNCATED AFTER RESIDUE 367 AND A HEXAHIS TAG ADDED. Source: (gene. exp.)   TRICHOPLUSIA NI (cabbage looper) / References: UniProt: P07700 TRICHOPLUSIA NI (cabbage looper) / References: UniProt: P07700#2: Chemical | #3: Sugar | ChemComp-SOG / | Compound details | ENGINEERED RESIDUE IN CHAIN A, ARG 68 TO SER ENGINEERED RESIDUE IN CHAIN A, MET 90 TO VAL ...ENGINEERED | Has protein modification | Y | Sequence details | THE FOLLOWING MUTATIONS WERE MADE TO IMPROVE THERMOSTABILITY R68S,M90V,Y227A,A282L,F327A,F338M. THE ...THE FOLLOWING MUTATIONS WERE MADE TO IMPROVE THERMOSTAB | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.48 Å3/Da / Density % sol: 64.64 % Description: DATA WERE COLLECTED IN WEDGES BY SCANNING FROM MULTIPLE SPOTS ON THE CRYSTAL |
|---|---|
| Crystal grow | pH: 7 / Details: pH 7.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-2 / Wavelength: 0.873 / Beamline: ID23-2 / Wavelength: 0.873 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Dec 14, 2007 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.873 Å / Relative weight: 1 |
| Reflection | Resolution: 3.65→41.3 Å / Num. obs: 11215 / % possible obs: 99.7 % / Observed criterion σ(I): 2 / Redundancy: 3.7 % / Biso Wilson estimate: 71.5 Å2 / Rmerge(I) obs: 0.1 / Net I/σ(I): 9.6 |
| Reflection shell | Resolution: 3.65→3.71 Å / Redundancy: 3.7 % / Rmerge(I) obs: 0.51 / Mean I/σ(I) obs: 3.3 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2VT4 Resolution: 3.65→41.31 Å / Cor.coef. Fo:Fc: 0.894 / Cor.coef. Fo:Fc free: 0.833 / SU B: 32.783 / SU ML: 0.502 / Cross valid method: THROUGHOUT / ESU R Free: 0.667 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES REFINED INDIVIDUALLY.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 90.037 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.65→41.31 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj