+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2r0p | ||||||
|---|---|---|---|---|---|---|---|
| Title | K252c-soaked RebC | ||||||
 Components Components | RebC | ||||||
 Keywords Keywords | OXIDOREDUCTASE / flavin adenine dinucleotide / K252c / Monooxygenase | ||||||
| Function / homology |  Function and homology information Function and homology informationoxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen, NAD(P)H as one donor, and incorporation of one atom of oxygen / FAD binding / methyltransferase activity / methylation Similarity search - Function | ||||||
| Biological species |  Lechevalieria aerocolonigenes (bacteria) Lechevalieria aerocolonigenes (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Rigid Body Refinement / Resolution: 2.1 Å SYNCHROTRON / Rigid Body Refinement / Resolution: 2.1 Å | ||||||
 Authors Authors | Ryan, K.S. / Drennan, C.L. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.Usa / Year: 2007 Journal: Proc.Natl.Acad.Sci.Usa / Year: 2007Title: Crystallographic trapping in the rebeccamycin biosynthetic enzyme RebC Authors: Ryan, K.S. / Howard-Jones, A.R. / Hamill, M.J. / Elliott, S.J. / Walsh, C.T. / Drennan, C.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2r0p.cif.gz 2r0p.cif.gz | 121.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2r0p.ent.gz pdb2r0p.ent.gz | 89.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2r0p.json.gz 2r0p.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/r0/2r0p https://data.pdbj.org/pub/pdb/validation_reports/r0/2r0p ftp://data.pdbj.org/pub/pdb/validation_reports/r0/2r0p ftp://data.pdbj.org/pub/pdb/validation_reports/r0/2r0p | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2r0cSC  2r0gC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 59927.625 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Lechevalieria aerocolonigenes (bacteria) Lechevalieria aerocolonigenes (bacteria)Strain: ATCC 39243 / Gene: rbmD, rebC / Plasmid: pET28a / Species (production host): Escherichia coli / Production host:  |
|---|---|
| #2: Chemical | ChemComp-CL / |
| #3: Chemical | ChemComp-FAD / |
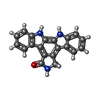
| #4: Chemical | ChemComp-K2C / |
| #5: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 50.73 % Description: No reasonable conformation for residues 417-425 could be built, despite the presence of electron density for this loop. Related structures 2R0C and 2R0P also show disorder in this ...Description: No reasonable conformation for residues 417-425 could be built, despite the presence of electron density for this loop. Related structures 2R0C and 2R0P also show disorder in this region, and the loop is likely to occupy multiple, overlapping orientations in this structure. |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.4 Details: 1.5 microliters of RebC (9 mg/mL in 150 mM NaCl, 10% glycerol, 25 mM HEPES pH 7.5) was incubated with 0.35 microliters of guanidine-HCl for 30 seconds, followed by addition of 1.5 ...Details: 1.5 microliters of RebC (9 mg/mL in 150 mM NaCl, 10% glycerol, 25 mM HEPES pH 7.5) was incubated with 0.35 microliters of guanidine-HCl for 30 seconds, followed by addition of 1.5 microliters of precipitant solution (19% PEG-8000, 0.1 M HEPES pH 7.4), without mixing, at room temperature and sealed over a precipitant well solution. Immediately after set up, crystal trays were placed on a gel shaker and then, after 12 hours, transferred to a storage space in vibration-isolation. A crystal was then soaked in 19% PEG-8000, 0.1 M HEPES pH 7.4, and 1 mM K252c for 11 days. The crystal was then soaked for 5 seconds in a cryogenic solution containing 19% PEG-8000, 0.1 M HEPES pH 7.4, 20% glycerol, and 1 mM K252c and then flash-frozen in liquid nitrogen., VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 141.4 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-C / Wavelength: 0.9795 Å / Beamline: 24-ID-C / Wavelength: 0.9795 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Mar 21, 2007 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→50 Å / Num. all: 31693 / Num. obs: 31693 / % possible obs: 91.9 % / Redundancy: 6.5 % / Rsym value: 0.053 / Net I/σ(I): 33.7 |
| Reflection shell | Resolution: 2.1→2.18 Å / Mean I/σ(I) obs: 3.2 / Num. unique all: 1854 / Rsym value: 0.291 / % possible all: 53.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: Rigid Body Refinement Starting model: PDB entry 2R0C Resolution: 2.1→50 Å / Isotropic thermal model: ISOTROPIC / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Displacement parameters | Biso mean: 56.3 Å2 | |||||||||||||||||||||||||
| Refine analyze |
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→50 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj