[English] 日本語
 Yorodumi
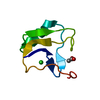
Yorodumi- PDB-2k57: Solution NMR Structure of Putative Lipoprotein from Pseudomonas s... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2k57 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Solution NMR Structure of Putative Lipoprotein from Pseudomonas syringae Gene Locus PSPTO2350. Northeast Structural Genomics Target PsR76A. | ||||||
 Components Components | Putative Lipoprotein | ||||||
 Keywords Keywords | structural genomics / unknown function / putative lipoprotein / PSI-2 / Protein Structure Initiative / Northeast Structural Genomics Consortium / NESG / Lipoprotein | ||||||
| Function / homology |  Function and homology information Function and homology informationProtein of unknown function DUF903 / : / Bacterial protein of unknown function (DUF903) / SH3 type barrels. - #100 / LSM domain superfamily / SH3 type barrels. / Prokaryotic membrane lipoprotein lipid attachment site profile. / Roll / Mainly Beta Similarity search - Domain/homology | ||||||
| Biological species |  Pseudomonas syringae pv. phaseolicola 1448A (bacteria) Pseudomonas syringae pv. phaseolicola 1448A (bacteria) | ||||||
| Method | SOLUTION NMR / torsion angle dynamics | ||||||
| Model details | putative lipoprotein truncated lipo-box | ||||||
 Authors Authors | Hang, D. / Aramini, J.A. / Rossi, P. / Wang, D. / Jiang, M. / Maglaqui, M. / Xiao, R. / Liu, J. / Baran, M.C. / Acton, T.B. ...Hang, D. / Aramini, J.A. / Rossi, P. / Wang, D. / Jiang, M. / Maglaqui, M. / Xiao, R. / Liu, J. / Baran, M.C. / Acton, T.B. / Rost, B. / Montelione, G.T. / Northeast Structural Genomics Consortium (NESG) | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Solution NMR Structure of Putative Lipoprotein from Pseudomonas syringae Gene Locus PSPTO2350. Northeast Structural Genomics Target PsR76A. Authors: Rossi, P. / Aramini, J.A. / Hang, D. / Xiao, R. / Acton, T.B. / Montelione, G.T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2k57.cif.gz 2k57.cif.gz | 382.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2k57.ent.gz pdb2k57.ent.gz | 321.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2k57.json.gz 2k57.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/k5/2k57 https://data.pdbj.org/pub/pdb/validation_reports/k5/2k57 ftp://data.pdbj.org/pub/pdb/validation_reports/k5/2k57 ftp://data.pdbj.org/pub/pdb/validation_reports/k5/2k57 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 7168.926 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Pseudomonas syringae pv. phaseolicola 1448A (bacteria) Pseudomonas syringae pv. phaseolicola 1448A (bacteria)Species: syringae / Gene: PSPPH_2109 / Plasmid: pET 21-23C / Species (production host): coli / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR details | Text: MONOMER BY GEL FILTRATION CHROMATOGRAPHY/LIGHT SCATTERING. STRUCTURE DETERMINED BY TRIPLE RESONANCE NMR SPECTROSCOPY. NOESY ASSIGNMENTS BY CYANA2.1. 20 OF 100 STRUCTURES LOWEST TARGET FUNCTION ...Text: MONOMER BY GEL FILTRATION CHROMATOGRAPHY/LIGHT SCATTERING. STRUCTURE DETERMINED BY TRIPLE RESONANCE NMR SPECTROSCOPY. NOESY ASSIGNMENTS BY CYANA2.1. 20 OF 100 STRUCTURES LOWEST TARGET FUNCTION SELECTED WITH CYANA2.1. SELECTED MODELS ARE FURTHER REFINED USING CNS IN EXPLICIT WATER SHELL (NILGES PROTOCOL BACKBONE 98.2%, SIDECHAIN 91.0%, AROMATIC (SC) 55.6%, VL METHYL STEREOSPECIFIC 100%, UNAMBIGUOS SIDECHAIN NH2 100%. STRUCTURE BASED ON 1027 NOE, 133 DIHE. MAX NOE VIOLATION: 0.14 A (1MODEL); MAX DIHE VIOLATION: 3.1 DEG. 2 TOTAL CLOSE ORDERED RESIDUES RANGES: 1-71 FOR [S(PHI)+S(PSI)] > 1.8. SECONDARY STRUCTURE - BETA STRANDS: (8-17, 20-26, 29-33, 51- 60, 65-70). RMSD 0.4 BACKBONE, 0.7 ALL HEAVY ATOMS. RAMA. CLASH: 11.98/-0.53 (RAW/Z). RPF SCORES ALL ASSIGNED RESIDUES PRECISION: 0.92, F-MEASURE: 0.95, DP-SCORE: 0.799. |
- Sample preparation
Sample preparation
| Details | Contents: 1.07 MM [U-100% 13C 15N] PSR76A, 50 UM DSS, 10 MM DTT, 100 MM SODIUM CHLORIDE, 0.02 % SODIUM AZIDE, 20 MM MES, 5 MM CALCIUM CHLORIDE, 90% H2O/10% D2O; 1.07 MM [5% 13C; U-100% 15N] PSR76A, ...Contents: 1.07 MM [U-100% 13C 15N] PSR76A, 50 UM DSS, 10 MM DTT, 100 MM SODIUM CHLORIDE, 0.02 % SODIUM AZIDE, 20 MM MES, 5 MM CALCIUM CHLORIDE, 90% H2O/10% D2O; 1.07 MM [5% 13C; U-100% 15N] PSR76A, 50 UM DSS, 10 MM DTT, 100 MM SODIUM CHLORIDE, 0.02 % SODIUM AZIDE, 20 MM MES, 5 MM CALCIUM CHLORIDE, 90% H2O/10% D2O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sample conditions | Ionic strength: 0.1 / pH: 6.5 / Pressure: AMBIENT / Temperature: 298 K |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: torsion angle dynamics / Software ordinal: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: target function / Conformers calculated total number: 100 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller











 PDBj
PDBj






 HSQC
HSQC