+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 2j9f | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Human branched-chain alpha-ketoacid dehydrogenase-decarboxylase E1b | ||||||
 要素 要素 | (2-OXOISOVALERATE DEHYDROGENASE ...) x 2 | ||||||
 キーワード キーワード | OXIDOREDUCTASE / FLAVOPROTEIN / MITOCHONDRION / THIAMINE PYROPHOSPHATE / MAPLE SYRUP URINE DISEASE / TRANSIT PEPTIDE / PHOSPHORYLATION / DISEASE MUTATION / METAL-BINDING / MULTI-ENZYME COMPLEX | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Loss-of-function mutations in BCKDHA or BCKDHB cause MSUD / 3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring) / branched-chain 2-oxo acid dehydrogenase activity / branched-chain alpha-ketoacid dehydrogenase complex / BCKDH synthesizes BCAA-CoA from KIC, KMVA, KIV / Loss-of-function mutations in DBT cause MSUD2 / Loss-of-function mutations in DLD cause MSUD3/DLDD / H139Hfs13* PPM1K causes a mild variant of MSUD / Branched-chain ketoacid dehydrogenase kinase deficiency / branched-chain amino acid catabolic process ...Loss-of-function mutations in BCKDHA or BCKDHB cause MSUD / 3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring) / branched-chain 2-oxo acid dehydrogenase activity / branched-chain alpha-ketoacid dehydrogenase complex / BCKDH synthesizes BCAA-CoA from KIC, KMVA, KIV / Loss-of-function mutations in DBT cause MSUD2 / Loss-of-function mutations in DLD cause MSUD3/DLDD / H139Hfs13* PPM1K causes a mild variant of MSUD / Branched-chain ketoacid dehydrogenase kinase deficiency / branched-chain amino acid catabolic process / Branched-chain amino acid catabolism / carboxy-lyase activity / response to glucocorticoid / response to cAMP / response to nutrient / lipid metabolic process / mitochondrial matrix / protein-containing complex binding / nucleolus / mitochondrion / nucleoplasm / metal ion binding 類似検索 - 分子機能 | ||||||
| 生物種 |  HOMO SAPIENS (ヒト) HOMO SAPIENS (ヒト) | ||||||
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  分子置換 / 解像度: 1.88 Å 分子置換 / 解像度: 1.88 Å | ||||||
 データ登録者 データ登録者 | Jun, L. / Machius, M. / Chuang, J.L. / Wynn, R.M. / Chuang, D.T. | ||||||
 引用 引用 |  ジャーナル: J.Biol.Chem. / 年: 2007 ジャーナル: J.Biol.Chem. / 年: 2007タイトル: The Two Active Sites in Human Branched-Chain Alpha- Keto Acid Dehydrogenase Operate Independently without an Obligatory Alternating-Site Mechanism. 著者: Li, J. / Machius, M. / Chuang, J.L. / Wynn, R.M. / Chuang, D.T. #1:  ジャーナル: J.Biol.Chem. / 年: 2004 ジャーナル: J.Biol.Chem. / 年: 2004タイトル: Cross-Talk between Thiamin Diphosphate Binding and Phosphorylation Loop Conformation in Human Branched-Chain Alpha-Keto Acid Decarboxylase/Dehydrogenase. 著者: Li, J. / Wynn, R.M. / Machius, M. / Chuang, J.L. / Karthikeyan, S. / Tomchick, D.R. / Chuang, D.T. #2: ジャーナル: J.Biol.Chem. / 年: 2001 タイトル: Roles of Active Site and Novel K+ Ion-Binding Site Residues in Human Mitochondrial Branched-Chain Alpha-Ketoacid Decarboxylase/Dehydrogenase. 著者: Wynn, R.M. / Ho, R. / Chuang, J.L. / Chuang, D.T. #3:  ジャーナル: Structure / 年: 2006 ジャーナル: Structure / 年: 2006タイトル: A Versatile Conformational Switch Regulates Reactivity in Human Branched-Chain Alpha-Ketoacid Dehydrogenase 著者: Machius, M. / Wynn, R.M. / Chuang, J.L. / Li, J. / Kluger, R. / Yu, D. / Tomchick, D.R. / Brautigam, C.A. / Chuang, D.T. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  2j9f.cif.gz 2j9f.cif.gz | 320.4 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb2j9f.ent.gz pdb2j9f.ent.gz | 257 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  2j9f.json.gz 2j9f.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/j9/2j9f https://data.pdbj.org/pub/pdb/validation_reports/j9/2j9f ftp://data.pdbj.org/pub/pdb/validation_reports/j9/2j9f ftp://data.pdbj.org/pub/pdb/validation_reports/j9/2j9f | HTTPS FTP |
|---|
-関連構造データ
| 関連構造データ |  1olsS S: 精密化の開始モデル |
|---|---|
| 類似構造データ |
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 単位格子 |
|
- 要素
要素
-2-OXOISOVALERATE DEHYDROGENASE ... , 2種, 4分子 ACBD
| #1: タンパク質 | 分子量: 45581.133 Da / 分子数: 2 / 変異: YES / 由来タイプ: 組換発現 / 由来: (組換発現)  HOMO SAPIENS (ヒト) / プラスミド: PTRC-ALPHA-BETAHIS / 発現宿主: HOMO SAPIENS (ヒト) / プラスミド: PTRC-ALPHA-BETAHIS / 発現宿主:  参照: UniProt: P12694, 3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring) #2: タンパク質 | 分子量: 38845.242 Da / 分子数: 2 / 由来タイプ: 組換発現 / 由来: (組換発現)  HOMO SAPIENS (ヒト) / プラスミド: PTRC-ALPHA-BETAHIS / 発現宿主: HOMO SAPIENS (ヒト) / プラスミド: PTRC-ALPHA-BETAHIS / 発現宿主:  参照: UniProt: P21953, 3-methyl-2-oxobutanoate dehydrogenase (2-methylpropanoyl-transferring) |
|---|
-非ポリマー , 5種, 1080分子 

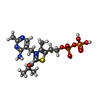






| #3: 化合物 | | #4: 化合物 | ChemComp-K / #5: 化合物 | #6: 化合物 | #7: 水 | ChemComp-HOH / | |
|---|
-詳細
| 構成要素の詳細 | ENGINEERED |
|---|
-実験情報
-実験
| 実験 | 手法:  X線回折 / 使用した結晶の数: 1 X線回折 / 使用した結晶の数: 1 |
|---|
- 試料調製
試料調製
| 結晶 | マシュー密度: 2.5 Å3/Da / 溶媒含有率: 50.7 % |
|---|---|
| 結晶化 | 温度: 293 K / 手法: 蒸気拡散法 / pH: 5.5 詳細: CRYSTALS WERE GROWN AT 20C VIA THE VAPOR DIFFUSION METHOD BY MIXING EQUAL AMOUNTS OF PROTEIN (20-25 MG/ML IN 50 MM HEPES/NAOH, PH 7.5, 250 MM KCL, 0.5 MM PMSF, 1 MM BENZAMIDINE AND 5% (V/V) ...詳細: CRYSTALS WERE GROWN AT 20C VIA THE VAPOR DIFFUSION METHOD BY MIXING EQUAL AMOUNTS OF PROTEIN (20-25 MG/ML IN 50 MM HEPES/NAOH, PH 7.5, 250 MM KCL, 0.5 MM PMSF, 1 MM BENZAMIDINE AND 5% (V/V) GLYCEROL) WITH WELL SOLUTION (1.4-1.6 M AMMONIUM SULFATE, 0.1 M NA-CITRATE PH 5.8, 20 MM B-MERCAPTOETHANOL). SERIALLY DILUTED CRUSHED CRYSTALS WERE USED FOR MICRO-SEEDING ONE DAY AFTER THE DROPS WERE SET UP. CRYSTALS APPEARED ONE DAY AFTER SEEDING AND GREW TO A MAXIMUM SIZE OF 120 X 800 UM WITHIN 10 DAYS. CRYSTALS WERE STABILIZED FOR 12 HOURS BY TRANSFER TO FRESH WELL SOLUTION. THEY WERE THEN CRYO-PROTECTED BY STEP-WISE TRANSFER INTO CRYO-BUFFER CONTAINING 1.6 M AMMONIUM SULFATE, 50 MM HEPES, PH 7.5, 100 MM NA-CITRATE, PH 5.8, 100 MM KCL, 50 MM DTT AND UP TO 20% (V/V) GLYCEROL. IT WAS FOUND THAT MANGANESE IONS COULD REPLACE THE MAGNESIUM REQUIRED FOR THE BINDING OF THDP TO THE ENZYME. |
-データ収集
| 回折 | 平均測定温度: 100 K |
|---|---|
| 放射光源 | 由来:  シンクロトロン / サイト: シンクロトロン / サイト:  APS APS  / ビームライン: 19-BM / 波長: 1.0062 / ビームライン: 19-BM / 波長: 1.0062 |
| 検出器 | タイプ: ADSC CCD / 検出器: CCD / 日付: 2006年7月14日 |
| 放射 | プロトコル: SINGLE WAVELENGTH / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray |
| 放射波長 | 波長: 1.0062 Å / 相対比: 1 |
| 反射 | 解像度: 1.88→26.64 Å / Num. obs: 135282 / % possible obs: 99.3 % / Observed criterion σ(I): -3 / 冗長度: 6.1 % / Biso Wilson estimate: 15.63 Å2 / Rmerge(I) obs: 0.1 / Net I/σ(I): 15.4 |
| 反射 シェル | 解像度: 1.88→1.91 Å / 冗長度: 6.2 % / Rmerge(I) obs: 0.66 / Mean I/σ(I) obs: 2.4 / % possible all: 100 |
- 解析
解析
| ソフトウェア |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 構造決定の手法:  分子置換 分子置換開始モデル: PDB ENTRY 1OLS 解像度: 1.88→26.64 Å / Cor.coef. Fo:Fc: 0.958 / Cor.coef. Fo:Fc free: 0.938 / SU B: 2.634 / SU ML: 0.079 / 交差検証法: THROUGHOUT / ESU R: 0.126 / ESU R Free: 0.117 / 立体化学のターゲット値: MAXIMUM LIKELIHOOD / 詳細: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 溶媒の処理 | イオンプローブ半径: 0.8 Å / 減衰半径: 0.8 Å / VDWプローブ半径: 1.4 Å / 溶媒モデル: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子変位パラメータ | Biso mean: 16.82 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: LAST / 解像度: 1.88→26.64 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 |
|
 ムービー
ムービー コントローラー
コントローラー



































 PDBj
PDBj





