[English] 日本語
 Yorodumi
Yorodumi- PDB-1yat: IMPROVED CALCINEURIN INHIBITION BY YEAST FKBP12-DRUG COMPLEXES. C... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1yat | ||||||
|---|---|---|---|---|---|---|---|
| Title | IMPROVED CALCINEURIN INHIBITION BY YEAST FKBP12-DRUG COMPLEXES. CRYSTALLOGRAPHIC AND FUNCTIONAL ANALYSIS | ||||||
 Components Components | FK506 BINDING PROTEIN | ||||||
 Keywords Keywords | BINDING PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of homoserine biosynthetic process / Calcineurin activates NFAT / negative regulation of homoserine biosynthetic process / : / macrolide binding / mTORC1-mediated signalling / nonfunctional rRNA decay / RNA polymerase II CTD heptapeptide repeat P3 isomerase activity / RNA polymerase II CTD heptapeptide repeat P6 isomerase activity / protein folding chaperone ...regulation of homoserine biosynthetic process / Calcineurin activates NFAT / negative regulation of homoserine biosynthetic process / : / macrolide binding / mTORC1-mediated signalling / nonfunctional rRNA decay / RNA polymerase II CTD heptapeptide repeat P3 isomerase activity / RNA polymerase II CTD heptapeptide repeat P6 isomerase activity / protein folding chaperone / peptidyl-prolyl cis-trans isomerase activity / peptidylprolyl isomerase / protein folding / chromatin organization / DNA-binding transcription activator activity, RNA polymerase II-specific / transcription by RNA polymerase II / mitochondrion / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.5 Å X-RAY DIFFRACTION / Resolution: 2.5 Å | ||||||
 Authors Authors | Rotonda, J. / Becker, J.W. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 1993 Journal: J.Biol.Chem. / Year: 1993Title: Improved calcineurin inhibition by yeast FKBP12-drug complexes. Crystallographic and functional analysis. Authors: Rotonda, J. / Burbaum, J.J. / Chan, H.K. / Marcy, A.I. / Becker, J.W. #1:  Journal: J.Biol.Chem. / Year: 1993 Journal: J.Biol.Chem. / Year: 1993Title: Fk-506-Binding Protein: Three-Dimensional Structure of the Complex with the Antagonist L-685,818 Authors: Becker, J.W. / Rotonda, J. / Mckeever, B.M. / Chan, H.K. / Marcy, A.I. / Wiederrecht, G. / Hermes, J.D. / Springer, J.P. | ||||||
| History |
| ||||||
| Remark 700 | SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE ...SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE SHEET RECORDS BELOW, TWO SIX-STRANDED SHEETS ARE DEFINED. STRANDS 1, 2, 3, 4, AND 5 ARE IDENTICAL IN BOTH SHEETS WHILE STRAND 6 IS DIFFERENT. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1yat.cif.gz 1yat.cif.gz | 35.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1yat.ent.gz pdb1yat.ent.gz | 23.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1yat.json.gz 1yat.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1yat_validation.pdf.gz 1yat_validation.pdf.gz | 838.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1yat_full_validation.pdf.gz 1yat_full_validation.pdf.gz | 843.8 KB | Display | |
| Data in XML |  1yat_validation.xml.gz 1yat_validation.xml.gz | 8.5 KB | Display | |
| Data in CIF |  1yat_validation.cif.gz 1yat_validation.cif.gz | 10.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ya/1yat https://data.pdbj.org/pub/pdb/validation_reports/ya/1yat ftp://data.pdbj.org/pub/pdb/validation_reports/ya/1yat ftp://data.pdbj.org/pub/pdb/validation_reports/ya/1yat | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 12038.628 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  References: UniProt: P20081 |
|---|---|
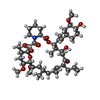
| #2: Chemical | ChemComp-FK5 / |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.11 Å3/Da / Density % sol: 70.09 % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 8.75 / Method: vapor diffusion | ||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Reflection | *PLUS Highest resolution: 2.5 Å / Num. obs: 6822 / % possible obs: 98 % / Num. measured all: 57469 / Rmerge(I) obs: 0.0977 |
|---|
- Processing
Processing
| Software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.5→8 Å / σ(F): 0 /
| ||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→8 Å
| ||||||||||||
| Refinement | *PLUS Highest resolution: 2.5 Å / Lowest resolution: 8 Å / Num. reflection all: 6822 / σ(F): 0 / Rfactor all: 0.177 | ||||||||||||
| Solvent computation | *PLUS | ||||||||||||
| Displacement parameters | *PLUS | ||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj