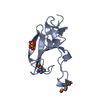
Entry Database : PDB / ID : 1w16Title rat synaptotagmin 4 C2B domain in the absence of calcium SYNAPTOTAGMIN IV Keywords / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species RATTUS NORVEGICUS (Norway rat)Method / / / Resolution : 2.3 Å Authors Dai, H. / Shin, O.-H. / Machius, M. / Tomchick, D.R. / Sudhof, T.C. / Rizo, J. Journal : Nat.Struct.Mol.Biol. / Year : 2004Title : Structural Basis for the Evolutionary Inactivation of Ca2+ Binding to Synaptotagmin 4Authors : Dai, H. / Shin, O.-H. / Machius, M. / Tomchick, D.R. / Sudhof, T.C. / Rizo, J. History Deposition Jun 16, 2004 Deposition site / Processing site Revision 1.0 Aug 13, 2004 Provider / Type Revision 1.1 Jul 13, 2011 Group / Version format complianceRevision 1.2 May 8, 2019 Group / Experimental preparation / OtherCategory database_PDB_rev / database_PDB_rev_record ... database_PDB_rev / database_PDB_rev_record / exptl_crystal_grow / pdbx_database_proc / pdbx_database_status Item / _pdbx_database_status.recvd_author_approvalRevision 1.3 Dec 13, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Other / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_database_status / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_sf / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å
MOLECULAR REPLACEMENT / Resolution: 2.3 Å  Authors
Authors Citation
Citation Journal: Nat.Struct.Mol.Biol. / Year: 2004
Journal: Nat.Struct.Mol.Biol. / Year: 2004 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 1w16.cif.gz
1w16.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb1w16.ent.gz
pdb1w16.ent.gz PDB format
PDB format 1w16.json.gz
1w16.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 1w16_validation.pdf.gz
1w16_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 1w16_full_validation.pdf.gz
1w16_full_validation.pdf.gz 1w16_validation.xml.gz
1w16_validation.xml.gz 1w16_validation.cif.gz
1w16_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/w1/1w16
https://data.pdbj.org/pub/pdb/validation_reports/w1/1w16 ftp://data.pdbj.org/pub/pdb/validation_reports/w1/1w16
ftp://data.pdbj.org/pub/pdb/validation_reports/w1/1w16

 Links
Links Assembly
Assembly
 Components
Components

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 19-BM / Wavelength: 0.9876
/ Beamline: 19-BM / Wavelength: 0.9876  Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller












 PDBj
PDBj






