[English] 日本語
 Yorodumi
Yorodumi- PDB-1tcc: THE SEQUENCE, CRYSTAL STRUCTURE DETERMINATION AND REFINEMENT OF T... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1tcc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | THE SEQUENCE, CRYSTAL STRUCTURE DETERMINATION AND REFINEMENT OF TWO CRYSTAL FORMS OF LIPASE B FROM CANDIDA ANTARCTICA | |||||||||
 Components Components | LIPASE | |||||||||
 Keywords Keywords | HYDROLASE(CARBOXYLIC ESTERASE) | |||||||||
| Function / homology |  Function and homology information Function and homology informationtriacylglycerol lipase / triacylglycerol lipase activity / lipid catabolic process Similarity search - Function | |||||||||
| Biological species |  Candida antarctica (fungus) Candida antarctica (fungus) | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.5 Å X-RAY DIFFRACTION / Resolution: 2.5 Å | |||||||||
 Authors Authors | Uppenberg, J. / Jones, T.A. | |||||||||
 Citation Citation |  Journal: Structure / Year: 1994 Journal: Structure / Year: 1994Title: The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Authors: Uppenberg, J. / Hansen, M.T. / Patkar, S. / Jones, T.A. #1:  Journal: J.Mol.Biol. / Year: 1994 Journal: J.Mol.Biol. / Year: 1994Title: Crystallization and Preliminary X-Ray Studies of Lipase B from Candida Antarctica Authors: Uppenberg, J. / Patkar, S. / Bergfors, T. / Jones, T.A. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1tcc.cif.gz 1tcc.cif.gz | 130.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1tcc.ent.gz pdb1tcc.ent.gz | 100.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1tcc.json.gz 1tcc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tc/1tcc https://data.pdbj.org/pub/pdb/validation_reports/tc/1tcc ftp://data.pdbj.org/pub/pdb/validation_reports/tc/1tcc ftp://data.pdbj.org/pub/pdb/validation_reports/tc/1tcc | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 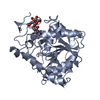
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO A 70 / 2: CIS PROLINE - PRO A 192 / 3: CIS PROLINE - PRO B 70 / 4: CIS PROLINE - PRO B 192 | ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (0.2813, 0.9354, 0.2141), Vector: Details | THIS IS THE HIGH PH STRUCTURE OF THE MONOCLINIC CRYSTAL; FORM WITH TWO MOLECULES IN THE ASYMMETRIC UNIT. THE MOLECULES ARE CALLED A AND B. THE RMS DEVIATIONS ARE 0.23 ANGSTROMS FOR C-ALPHAS AND 0.33 ANGSTROMS FOR ALL ATOMS. THE TRANSFORMATION PRESENTED ON *MTRIX* RECORDS BELOW WILL YIELD APPROXIMATE COORDINATES FOR CHAIN B WHEN APPLIED TO CHAIN A. | |
- Components
Components
| #1: Protein | Mass: 33040.238 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Candida antarctica (fungus) / References: UniProt: P41365, triacylglycerol lipase Candida antarctica (fungus) / References: UniProt: P41365, triacylglycerol lipase#2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #3: Sugar | ChemComp-BOG / | #4: Water | ChemComp-HOH / | Has protein modification | Y | Sequence details | THE SEQUENCE HAS NOT BEEN REPORTED. IT WAS DERIVED FROM THE DNA SEQUENCE OF J.UPPENBERG ET AL., ...THE SEQUENCE HAS NOT BEEN REPORTED. IT WAS DERIVED FROM THE DNA SEQUENCE OF J.UPPENBERG ET AL., 1994, LISTED IN THE JRNL REFERENCE ABOVE. | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.18 Å3/Da / Density % sol: 43.66 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 3.6 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Reflection | *PLUS Highest resolution: 2.5 Å / Num. obs: 18925 / % possible obs: 94 % / Num. measured all: 48771 / Rmerge(I) obs: 0.056 |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.5→7.5 Å / σ(F): 2 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→7.5 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.196 / Rfactor Rwork: 0.196 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_d / Dev ideal: 1.3 |
 Movie
Movie Controller
Controller













 PDBj
PDBj


