+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1qd6 | ||||||
|---|---|---|---|---|---|---|---|
| Title | OUTER MEMBRANE PHOSPHOLIPASE A FROM ESCHERICHIA COLI | ||||||
 Components Components |
| ||||||
 Keywords Keywords | MEMBRANE PROTEIN / ANTI-PARALLEL BETA BARREL DIMER | ||||||
| Function / homology |  Function and homology information Function and homology informationphospholipase A1 / glycerophospholipase activity / glycerophospholipid phospholipase A1 activity / phosphatidylglycerol metabolic process / A2-type glycerophospholipase activity / phospholipase A2 / phosphatidylcholine lysophospholipase A1 activity / lipid catabolic process / cell outer membrane / calcium ion binding / protein homodimerization activity Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2.1 Å SYNCHROTRON / Resolution: 2.1 Å | ||||||
 Authors Authors | Snijder, H.J. / Ubarretxena-Belandia, I. / Blaauw, M. / Kalk, K.H. / Verheij, H.M. / Egmond, M.R. / Dekker, N. / Dijkstra, B.W. | ||||||
 Citation Citation |  Journal: Nature / Year: 1999 Journal: Nature / Year: 1999Title: Structural evidence for dimerization-regulated activation of an integral membrane phospholipase. Authors: Snijder, H.J. / Ubarretxena-Belandia, I. / Blaauw, M. / Kalk, K.H. / Verheij, H.M. / Egmond, M.R. / Dekker, N. / Dijkstra, B.W. #1:  Journal: FEBS Lett. / Year: 1995 Journal: FEBS Lett. / Year: 1995Title: Crystallization and preliminary X-ray analysis of outer membrane phospholipase A from Escherichia coli Authors: Blaauw, M. / Dekker, N. / Kalk, K.H. / Verheij, H.M. / Dijkstra, B.W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1qd6.cif.gz 1qd6.cif.gz | 116 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1qd6.ent.gz pdb1qd6.ent.gz | 89.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1qd6.json.gz 1qd6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qd/1qd6 https://data.pdbj.org/pub/pdb/validation_reports/qd/1qd6 ftp://data.pdbj.org/pub/pdb/validation_reports/qd/1qd6 ftp://data.pdbj.org/pub/pdb/validation_reports/qd/1qd6 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1qd5SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 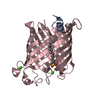
| ||||||||
| 2 | 
| ||||||||
| 3 |
| ||||||||
| Unit cell |
| ||||||||
| Details | The biological assembly is a dimer constructed from chain A,C and B,D related by a NCS twofold rotation |
- Components
Components
| #1: Protein/peptide | Mass: 1402.640 Da / Num. of mol.: 2 / Fragment: RESDIUES 33-45 / Source method: obtained synthetically / References: UniProt: P0A921 #2: Protein | Mass: 27713.598 Da / Num. of mol.: 2 Mutation: ENZYME WITH N-TERMINAL EXTENSION ARIRAP AND COVALENTLY SULFONYLATED ON SERINE144 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #3: Chemical | #4: Chemical | #5: Water | ChemComp-HOH / | Has protein modification | Y | Sequence details | THE N-TERMINAL WAS MUTATED TO EXTEND WITH RESIDUES ARIRAP | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.75 Å3/Da / Density % sol: 55.28 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 6.6 Details: PEG400, pH 6.6, VAPOR DIFFUSION, HANGING DROP, temperature 20K | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 6.5 / Method: vapor diffusionDetails: protein solution is mixed in a 3:2 ratio with well solution | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 120 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID2 / Wavelength: 0.99385 / Beamline: ID2 / Wavelength: 0.99385 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Sep 13, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.99385 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→20.3 Å / Num. all: 76223 / Num. obs: 28336 / % possible obs: 74.1 % / Redundancy: 2.69 % / Biso Wilson estimate: 27.1 Å2 / Rmerge(I) obs: 0.077 / Net I/σ(I): 10.9 |
| Reflection shell | Resolution: 2.1→2.17 Å / Rmerge(I) obs: 0.265 / Num. unique all: 2510 / % possible all: 66.7 |
| Reflection shell | *PLUS % possible obs: 66.7 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Starting model: MONOMER OMPLA (1QD5) WITHOUT WATER/DETERGENT MOLECULES Resolution: 2.1→20.3 Å / Rfactor Rfree error: 0.006 / Data cutoff high absF: 100000 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber / Details: Bulk solvent correction Anisotropic scaling
| ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 34.5 Å2
| ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→20.3 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.1→2.17 Å / Rfactor Rfree error: 0.021 / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.843 / Classification: refinement X-PLOR / Version: 3.843 / Classification: refinement | ||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj






