[English] 日本語
 Yorodumi
Yorodumi- PDB-1pth: The Structural Basis of Aspirin Activity Inferred from the Crysta... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1pth | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The Structural Basis of Aspirin Activity Inferred from the Crystal Structure of Inactivated Prostaglandin H2 Synthase | |||||||||
 Components Components | PROSTAGLANDIN H2 SYNTHASE-1 | |||||||||
 Keywords Keywords | OXIDOREDUCTASE / DIOXYGENASE / PEROXIDASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationprostaglandin-endoperoxide synthase / prostaglandin-endoperoxide synthase activity / cyclooxygenase pathway / oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen / prostaglandin biosynthetic process / peroxidase activity / regulation of blood pressure / response to oxidative stress / neuron projection / heme binding ...prostaglandin-endoperoxide synthase / prostaglandin-endoperoxide synthase activity / cyclooxygenase pathway / oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen / prostaglandin biosynthetic process / peroxidase activity / regulation of blood pressure / response to oxidative stress / neuron projection / heme binding / endoplasmic reticulum membrane / protein homodimerization activity / metal ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 3.4 Å X-RAY DIFFRACTION / Resolution: 3.4 Å | |||||||||
 Authors Authors | Loll, P.J. / Picot, D. / Garavito, R.M. | |||||||||
 Citation Citation |  Journal: Nat.Struct.Biol. / Year: 1995 Journal: Nat.Struct.Biol. / Year: 1995Title: The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Authors: Loll, P.J. / Picot, D. / Garavito, R.M. #1:  Journal: Nature / Year: 1994 Journal: Nature / Year: 1994Title: The X-Ray Crystal Structure of the Membrane Protein Prostaglandin H2 Synthase-1 Authors: Picot, D. / Loll, P.J. / Garavito, R.M. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1pth.cif.gz 1pth.cif.gz | 237.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1pth.ent.gz pdb1pth.ent.gz | 191.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1pth.json.gz 1pth.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pt/1pth https://data.pdbj.org/pub/pdb/validation_reports/pt/1pth ftp://data.pdbj.org/pub/pdb/validation_reports/pt/1pth ftp://data.pdbj.org/pub/pdb/validation_reports/pt/1pth | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.995361, -0.058518, 0.076371), Vector: Details | MTRIX THE TRANSFORMATION PRESENTED ON MTRIX RECORDS BELOW DESCRIBES THE NON-CRYSTALLOGRAPHIC RELATIONSHIP TO CREATE THE SECOND SUBUNIT OF THE PGHS-1 DIMER. | |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 66285.742 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  References: PIR: A29947, UniProt: P05979*PLUS, prostaglandin-endoperoxide synthase |
|---|
-Sugars , 3 types, 8 molecules 


| #2: Polysaccharide | Source method: isolated from a genetically manipulated source #3: Sugar | ChemComp-NAG / #4: Sugar | |
|---|
-Non-polymers , 3 types, 5 molecules 
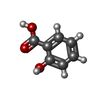



| #5: Chemical | | #6: Chemical | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Compound details | COMPND MOLECULE: PROSTAGLANDIN H2 SYNTHASE-1. ACETYLATED BY THE ASPIRIN ANALOG 2-BROMOACETOXY ...COMPND MOLECULE: PROSTAGLAN |
|---|---|
| Has protein modification | Y |
| Sequence details | THIS SEQUENCE IS FOUND IN ENTRY 1PRH. THE NUMBERING IS DERIVED FROM THE PRE-PROTEIN, WHICH CONTAINS ...THIS SEQUENCE IS FOUND IN ENTRY 1PRH. THE NUMBERING IS DERIVED FROM THE PRE-PROTEIN, WHICH CONTAINS A 24-RESIDUE SIGNAL SEQUENCE WHICH IS CLEAVED DURING MATURATION |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.63 Å3/Da / Density % sol: 73.46 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | *PLUS Density % sol: 71 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 6.7 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Detector | Date: Jun 1, 1993 |
|---|---|
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Relative weight: 1 |
| Reflection | Resolution: 3.4→15 Å / Num. obs: 27154 / % possible obs: 82 % / Observed criterion σ(I): 1 / Redundancy: 2.7 % / Rmerge(I) obs: 0.095 |
| Reflection | *PLUS Num. measured all: 68275 / Rmerge(I) obs: 0.095 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 3.4→8 Å / σ(F): 1
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 21 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.4→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj







