[English] 日本語
 Yorodumi
Yorodumi- PDB-1odu: CRYSTAL STRUCTURE OF THERMOTOGA MARITIMA ALPHA-FUCOSIDASE IN COMP... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1odu | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF THERMOTOGA MARITIMA ALPHA-FUCOSIDASE IN COMPLEX WITH FUCOSE | ||||||
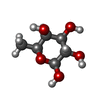
 Components Components | PUTATIVE ALPHA-L-FUCOSIDASE | ||||||
 Keywords Keywords | HYDROLASE / GLYCOSIDE HYDROLASE / ALPHA-L-FUCOSIDASE / PRODUCT COMPLEX / THERMOSTABLE | ||||||
| Function / homology |  Function and homology information Function and homology informationalpha-L-fucosidase activity / fucose metabolic process / glycoside catabolic process / lysosome Similarity search - Function | ||||||
| Biological species |   THERMOTOGA MARITIMA (bacteria) THERMOTOGA MARITIMA (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.8 Å MOLECULAR REPLACEMENT / Resolution: 2.8 Å | ||||||
 Authors Authors | Sulzenbacher, G. / Bignon, C. / Bourne, Y. / Henrissat, B. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2004 Journal: J.Biol.Chem. / Year: 2004Title: Crystal Structure of Thermotoga Maritima {Alpha}-L-Fucosidase: Insights Into the Catalytic Mechanism and the Molecular Basis for Fucosidosis Authors: Sulzenbacher, G. / Bignon, C. / Nishimura, T. / Tarling, C. / Withers, S. / Henrissat, B. / Bourne, Y. #1: Journal: J.Biol.Chem. / Year: 2003 Title: Identification of the Catalytic Nucleophile of the Family 29 Alpha -L-Fucosidase from Thermotoga Maritima Through Trapping of a Covalent Glycosyl-Enzyme Intermediate and Mutagenesis Authors: Tarling, C. / He, S. / Sulzenbacher, G. / Bignon, C. / Bourne, Y. / Henrissat, B. / Withers, S. | ||||||
| History |
| ||||||
| Remark 650 | HELIX DETERMINATION METHOD: AUTHOR PROVIDED. | ||||||
| Remark 700 | SHEET DETERMINATION METHOD: AUTHOR PROVIDED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1odu.cif.gz 1odu.cif.gz | 180.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1odu.ent.gz pdb1odu.ent.gz | 145.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1odu.json.gz 1odu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/od/1odu https://data.pdbj.org/pub/pdb/validation_reports/od/1odu ftp://data.pdbj.org/pub/pdb/validation_reports/od/1odu ftp://data.pdbj.org/pub/pdb/validation_reports/od/1odu | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1hl8SC  1hl9C S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.70016, -0.71398, -0.00014), Vector: Details | BETWEEN THE ISOLATED CHAINS AND THAT FOR THE CHAINSIN THE COMPLEX IS IN AVERAGE 2464 (+/- 52) ANGSTROM**2. | |
- Components
Components
| #1: Protein | Mass: 52272.051 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: ORF TM0306 / Source: (gene. exp.)   THERMOTOGA MARITIMA (bacteria) / Strain: MSB8 / Production host: THERMOTOGA MARITIMA (bacteria) / Strain: MSB8 / Production host:  #2: Sugar | Compound details | ALPHA-L-FUCOSIDASE BELONGS TO GLYCOSYL HYDROLASASE FAMILY 29. THE ENZYME PERFORMS CATALYSIS WITH ...ALPHA-L-FUCOSIDASE | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.74 Å3/Da / Density % sol: 54.8 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 8 Details: 18% PEG600, 5% JEFFAMINE M-600, 100 MM TRIS-HCL PH 8.0, PROTEIN CONCENTRATION 5 MG/ML FUCOSE WAS INTRODUCED BY SHORT SOAKING OF A NATIVE | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 8 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-2 / Wavelength: 0.933 / Beamline: ID14-2 / Wavelength: 0.933 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Sep 16, 2001 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.933 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→57.7 Å / Num. obs: 24598 / % possible obs: 98.1 % / Redundancy: 3.9 % / Biso Wilson estimate: 100.81 Å2 / Rmerge(I) obs: 0.056 / Net I/σ(I): 6.8 |
| Reflection shell | Resolution: 2.8→2.87 Å / Redundancy: 3.9 % / Rmerge(I) obs: 0.442 / Mean I/σ(I) obs: 1.6 / % possible all: 98.1 |
| Reflection | *PLUS Highest resolution: 2.8 Å / Lowest resolution: 57 Å / Redundancy: 3.9 % / Num. measured all: 99148 / Rmerge(I) obs: 0.056 |
| Reflection shell | *PLUS % possible obs: 98.1 % / Redundancy: 3.9 % / Rmerge(I) obs: 0.442 / Mean I/σ(I) obs: 1.6 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: NATIVE ALPHA-L-FUCOSIDASE, PDB ENTRY 1HL8 Resolution: 2.8→19.76 Å / Cor.coef. Fo:Fc: 0.954 / Cor.coef. Fo:Fc free: 0.942 / SU B: 14.034 / SU ML: 0.274 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / ESU R Free: 0.36 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. SOLVENT NO WATER MOLECULES HAVE BEEN ADDED GIVEN THE MODEST RESOLUTION AND THE HIGH OVERALL DISORDER OF THE STRUCTURE.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.8→19.76 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj