+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ne5 | ||||||
|---|---|---|---|---|---|---|---|
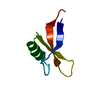
| Title | Solution Structure of HERG Specific Scorpion Toxin CnErg1 | ||||||
 Components Components | ergtoxin | ||||||
 Keywords Keywords | TOXIN / alpha-helix / triple-stranded beta-sheet | ||||||
| Function / homology |  Function and homology information Function and homology informationpotassium channel inhibitor activity / toxin activity / extracellular region Similarity search - Function | ||||||
| Method | SOLUTION NMR / simulated annealing, molecular dynamics, distance geometry, torsion angle dynamics | ||||||
 Authors Authors | Torres, A.M. / Paramjit, B. / Alewood, P. / Kuchel, P.W. / Vandenberg, J.I. | ||||||
 Citation Citation |  Journal: FEBS Lett. / Year: 2003 Journal: FEBS Lett. / Year: 2003Title: Solution structure of CnErg1 (Ergtoxin), a HERG specific scorpion toxin Authors: Torres, A.M. / Bansal, P. / Alewood, P.F. / Bursill, J.A. / Kuchel, P.W. / Vandenberg, J.I. #1:  Journal: FASEB J. / Year: 1999 Journal: FASEB J. / Year: 1999Title: A toxin to nervous, cardiac, and endocrine ERG K+ channels isolated from Centruroides noxius scorpion venom. Authors: Gurrola, G.B. / Rosati, B. / Rocchetti, M. / Pimienta, G. / Zaza, A. / Arcangeli, A. / Olivotto, M. / Possani, L.D. / Wanke, E. #2:  Journal: FEBS Lett. / Year: 2000 Journal: FEBS Lett. / Year: 2000Title: Disulfide bridges of ergtoxin, a member of a new sub-family of peptide blockers of the ether-a-go-go-related K+ channel Authors: Scaloni, A. / Bottiglieri, C. / Ferrara, L. / Corona, M. / Gurrola, G.B. / Batista, C. / Wanke, E. / Possani, L.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ne5.cif.gz 1ne5.cif.gz | 241.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ne5.ent.gz pdb1ne5.ent.gz | 200.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ne5.json.gz 1ne5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1ne5_validation.pdf.gz 1ne5_validation.pdf.gz | 338.2 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1ne5_full_validation.pdf.gz 1ne5_full_validation.pdf.gz | 462.5 KB | Display | |
| Data in XML |  1ne5_validation.xml.gz 1ne5_validation.xml.gz | 13.3 KB | Display | |
| Data in CIF |  1ne5_validation.cif.gz 1ne5_validation.cif.gz | 21.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ne/1ne5 https://data.pdbj.org/pub/pdb/validation_reports/ne/1ne5 ftp://data.pdbj.org/pub/pdb/validation_reports/ne/1ne5 ftp://data.pdbj.org/pub/pdb/validation_reports/ne/1ne5 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 4746.415 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: This sequence occurs naturally in Centruroides noxius References: UniProt: Q86QT3 |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|---|
| NMR experiment | Type: 2D NOESY |
| NMR details | Text: This structure were determined using standard 2D homonuclear techniques. |
- Sample preparation
Sample preparation
| Details | Contents: 1.7mM CnErg1 / Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample conditions | Ionic strength: no salt added / pH: 3.2 / Pressure: ambient / Temperature: 298 K |
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| NMR spectrometer | Type: Bruker AVANCE / Manufacturer: Bruker / Model: AVANCE / Field strength: 600 MHz |
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: simulated annealing, molecular dynamics, distance geometry, torsion angle dynamics Software ordinal: 1 Details: The structures are based on a total of 578 restraints, 535 are NOE-derived distance restraints, 11 dihedral angle restraints, 32 distance restraints from hydrogen bonds. | ||||||||||||||||||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 4000 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller









 PDBj
PDBj

