+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 1mfg | ||||||
|---|---|---|---|---|---|---|---|
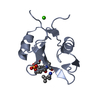
| タイトル | The Structure of ERBIN PDZ domain bound to the Carboxy-terminal tail of the ErbB2 Receptor | ||||||
 要素 要素 |
| ||||||
 キーワード キーワード | SIGNALING PROTEIN / PDZ DOMAIN / PROTEIN-PEPTIDE COMPLEX / ERB-B2 / ERBIN. | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報ErbB-2 class receptor binding / basal protein localization / negative regulation of nucleotide-binding oligomerization domain containing 2 signaling pathway / hemidesmosome / negative regulation of immature T cell proliferation in thymus / ERBB3:ERBB2 complex / postsynaptic specialization / ERBB2-ERBB4 signaling pathway / GRB7 events in ERBB2 signaling / RNA polymerase I core binding ...ErbB-2 class receptor binding / basal protein localization / negative regulation of nucleotide-binding oligomerization domain containing 2 signaling pathway / hemidesmosome / negative regulation of immature T cell proliferation in thymus / ERBB3:ERBB2 complex / postsynaptic specialization / ERBB2-ERBB4 signaling pathway / GRB7 events in ERBB2 signaling / RNA polymerase I core binding / immature T cell proliferation in thymus / intermediate filament cytoskeleton organization / semaphorin receptor complex / negative regulation of monocyte chemotactic protein-1 production / establishment or maintenance of epithelial cell apical/basal polarity / regulation of microtubule-based process / ErbB-3 class receptor binding / motor neuron axon guidance / Sema4D induced cell migration and growth-cone collapse / PLCG1 events in ERBB2 signaling / RHOB GTPase cycle / ERBB2-EGFR signaling pathway / negative regulation of NF-kappaB transcription factor activity / ERBB2 Activates PTK6 Signaling / neurotransmitter receptor localization to postsynaptic specialization membrane / enzyme-linked receptor protein signaling pathway / neuromuscular junction development / positive regulation of Rho protein signal transduction / ERBB2-ERBB3 signaling pathway / RHOC GTPase cycle / Drug-mediated inhibition of ERBB2 signaling / Resistance of ERBB2 KD mutants to trastuzumab / Resistance of ERBB2 KD mutants to sapitinib / Resistance of ERBB2 KD mutants to tesevatinib / Resistance of ERBB2 KD mutants to neratinib / Resistance of ERBB2 KD mutants to osimertinib / Resistance of ERBB2 KD mutants to afatinib / Resistance of ERBB2 KD mutants to AEE788 / Resistance of ERBB2 KD mutants to lapatinib / Drug resistance in ERBB2 TMD/JMD mutants / positive regulation of MAP kinase activity / response to muramyl dipeptide / positive regulation of transcription by RNA polymerase I / ERBB2 Regulates Cell Motility / semaphorin-plexin signaling pathway / oligodendrocyte differentiation / basement membrane / PI3K events in ERBB2 signaling / RHOG GTPase cycle / RHOA GTPase cycle / positive regulation of protein targeting to membrane / RAC2 GTPase cycle / RAC3 GTPase cycle / regulation of angiogenesis / regulation of ERK1 and ERK2 cascade / protein targeting / Schwann cell development / regulation of postsynaptic membrane neurotransmitter receptor levels / coreceptor activity / Signaling by ERBB2 / RAC1 GTPase cycle / TFAP2 (AP-2) family regulates transcription of growth factors and their receptors / myelination / transmembrane receptor protein tyrosine kinase activity / GRB2 events in ERBB2 signaling / positive regulation of cell adhesion / SHC1 events in ERBB2 signaling / Downregulation of ERBB2:ERBB3 signaling / cell surface receptor protein tyrosine kinase signaling pathway / basal plasma membrane / Constitutive Signaling by Overexpressed ERBB2 / cellular response to epidermal growth factor stimulus / peptidyl-tyrosine phosphorylation / positive regulation of translation / positive regulation of epithelial cell proliferation / integrin-mediated signaling pathway / neuromuscular junction / phosphatidylinositol 3-kinase/protein kinase B signal transduction / wound healing / Signaling by ERBB2 TMD/JMD mutants / receptor protein-tyrosine kinase / Signaling by ERBB2 ECD mutants / receptor tyrosine kinase binding / Signaling by ERBB2 KD Mutants / cellular response to growth factor stimulus / structural constituent of cytoskeleton / ruffle membrane / Downregulation of ERBB2 signaling / epidermal growth factor receptor signaling pathway / neuron differentiation / Constitutive Signaling by Aberrant PI3K in Cancer / cellular response to tumor necrosis factor / cell junction / transmembrane signaling receptor activity / PIP3 activates AKT signaling / myelin sheath / heart development / presynaptic membrane / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / RAF/MAP kinase cascade 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  多波長異常分散 / 解像度: 1.25 Å 多波長異常分散 / 解像度: 1.25 Å | ||||||
 データ登録者 データ登録者 | Birrane, G. / Chung, J. / Ladias, J.A. | ||||||
 引用 引用 |  ジャーナル: J.Biol.Chem. / 年: 2003 ジャーナル: J.Biol.Chem. / 年: 2003タイトル: Novel mode of ligand recognition by the erbin PDZ domain 著者: Birrane, G. / Chung, J. / Ladias, J.A. #1:  ジャーナル: NAT.CELL BIOL. / 年: 2000 ジャーナル: NAT.CELL BIOL. / 年: 2000タイトル: BIN: A BASOLATERAL PDZ PROTEIN THAT INTERACTS WITH THE MAMMALIAN ERBB2/HER2 RECEPTOR 著者: BORG, J.P. / MARCHETTO, S. / LEBIVIC, A. / OLLENDORFF, V. / JAULIN-BASTARD, F. / SAITO, H. / FOURNIER, E. / ADELAIDE, J. / MARGOLIS, B. / BIRNBAUM, D. #2:  ジャーナル: J.Biol.Chem. / 年: 2002 ジャーナル: J.Biol.Chem. / 年: 2002タイトル: THE ERBIN PDZ DOMAIN BINDS WITH HIGH AFFINITY AND SPECIFICITY TO THE CARBOXYL TERMINI OF DELTA-CATENIN AND ARVCF 著者: LAURA, R.P. / WITT, A.S. / HELD, H.A. / GERSTNER, R. / DESHAYES, K. / KOEHLER, M.F. / KOSIK, K.S. / SIDHU, S.S. / LASKY, L.A. #3:  ジャーナル: J.Biol.Chem. / 年: 2001 ジャーナル: J.Biol.Chem. / 年: 2001タイトル: ERBIN IS A PROTEIN CONCENTRATED AT POSTSYNAPTIC MEMBRANES THAT INTERACTS WITH PSD-95 著者: HUANG, Y.Z. / WANG, Q. / XIONG, W.C. / MEI, L. #4:  ジャーナル: J.Biol.Chem. / 年: 2001 ジャーナル: J.Biol.Chem. / 年: 2001タイトル: THE ERBB2/HER2 RECEPTOR DIFFERENTIALLY INTERACTS WITH ERBIN AND PICK1 PSD-95/DLG/ZO-1 DOMAIN PROTEINS 著者: JAULIN-BASTARD, F. / SAITO, H. / LEBIVIC, A. / OLLENDORFF, V. / MARCHETTO, S. / BIRNBAUM, D. / BORG, J.P. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  1mfg.cif.gz 1mfg.cif.gz | 59.6 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb1mfg.ent.gz pdb1mfg.ent.gz | 43.7 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  1mfg.json.gz 1mfg.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  1mfg_validation.pdf.gz 1mfg_validation.pdf.gz | 420.8 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  1mfg_full_validation.pdf.gz 1mfg_full_validation.pdf.gz | 422.5 KB | 表示 | |
| XML形式データ |  1mfg_validation.xml.gz 1mfg_validation.xml.gz | 8.1 KB | 表示 | |
| CIF形式データ |  1mfg_validation.cif.gz 1mfg_validation.cif.gz | 10.8 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/mf/1mfg https://data.pdbj.org/pub/pdb/validation_reports/mf/1mfg ftp://data.pdbj.org/pub/pdb/validation_reports/mf/1mfg ftp://data.pdbj.org/pub/pdb/validation_reports/mf/1mfg | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 単位格子 |
| ||||||||
| 詳細 | COORDINATES REPRESENT THE COMPLETE BIOLOGICAL ASSEMBLY |
- 要素
要素
| #1: タンパク質 | 分子量: 10292.593 Da / 分子数: 1 / 断片: PDZ DOMAIN / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / プラスミド: PGEX-KT / 生物種 (発現宿主): Escherichia coli / 発現宿主: Homo sapiens (ヒト) / プラスミド: PGEX-KT / 生物種 (発現宿主): Escherichia coli / 発現宿主:  |
|---|---|
| #2: タンパク質・ペプチド | 分子量: 1004.134 Da / 分子数: 1 / 断片: PEPTIDE EYLGLDVPV / 由来タイプ: 合成 / 詳細: PEPTIDE SYNTHESIZED CHEMICALLY / 参照: UniProt: P04626*PLUS |
| #3: 水 | ChemComp-HOH / |
-実験情報
-実験
| 実験 | 手法:  X線回折 / 使用した結晶の数: 1 X線回折 / 使用した結晶の数: 1 |
|---|
- 試料調製
試料調製
| 結晶 | マシュー密度: 1.71 Å3/Da / 溶媒含有率: 39.16 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 結晶化 | 温度: 292 K / 手法: 蒸気拡散法, シッティングドロップ法 / pH: 4.6 詳細: 12-15%PEG 4000, 100mM Ammonium Acetate, 100mM Sodium Acetate, 10% Glycerol, pH 4.6, VAPOR DIFFUSION, SITTING DROP, temperature 292K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 結晶化 | *PLUS 温度: 20 ℃ / pH: 8.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 溶液の組成 | *PLUS
|
-データ収集
| 回折 | 平均測定温度: 100 K |
|---|---|
| 放射光源 | 由来:  シンクロトロン / サイト: シンクロトロン / サイト:  CHESS CHESS  / ビームライン: F2 / 波長: 0.97861 Å / ビームライン: F2 / 波長: 0.97861 Å |
| 検出器 | タイプ: ADSC QUANTUM 4 / 検出器: CCD / 日付: 2002年3月10日 / 詳細: DUAL SLITS |
| 放射 | モノクロメーター: SAGITALLY FOCUSED Si(111) / プロトコル: MAD / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray |
| 放射波長 | 波長: 0.97861 Å / 相対比: 1 |
| 反射 | 解像度: 1.25→25 Å / Num. all: 24313 / Num. obs: 24313 / % possible obs: 97.6 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 |
| 反射 シェル | 解像度: 1.25→1.29 Å / % possible all: 91.4 |
| 反射 | *PLUS % possible obs: 92.7 % / Rmerge(I) obs: 0.025 |
| 反射 シェル | *PLUS % possible obs: 91.4 % / Rmerge(I) obs: 0.037 / Mean I/σ(I) obs: 16.8 |
- 解析
解析
| ソフトウェア |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 構造決定の手法:  多波長異常分散 / 解像度: 1.25→25 Å / Num. parameters: 8637 / Num. restraintsaints: 10570 / 交差検証法: FREE R / σ(F): 0 / 立体化学のターゲット値: Engh & Huber 多波長異常分散 / 解像度: 1.25→25 Å / Num. parameters: 8637 / Num. restraintsaints: 10570 / 交差検証法: FREE R / σ(F): 0 / 立体化学のターゲット値: Engh & Huber詳細: ANISOTROPIC REFINEMENT REDUCED FREE R (NO CUTOFF) BY 2.6% Water molecules 131, 132 and 133 occupy the site where the disordered component of HIS1347 with alternate conformer B is modeled.
| |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 8 / Occupancy sum hydrogen: 766 / Occupancy sum non hydrogen: 929.08 | |||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: LAST / 解像度: 1.25→25 Å
| |||||||||||||||||||||||||||||||||
| 拘束条件 |
| |||||||||||||||||||||||||||||||||
| ソフトウェア | *PLUS 名称: SHELXL / バージョン: 97 / 分類: refinement | |||||||||||||||||||||||||||||||||
| 精密化 | *PLUS 最低解像度: 25 Å / % reflection Rfree: 5 % / Rfactor Rfree: 0.164 / Rfactor Rwork: 0.128 | |||||||||||||||||||||||||||||||||
| 溶媒の処理 | *PLUS | |||||||||||||||||||||||||||||||||
| 原子変位パラメータ | *PLUS | |||||||||||||||||||||||||||||||||
| 拘束条件 | *PLUS
|
 ムービー
ムービー コントローラー
コントローラー












 PDBj
PDBj
















