[English] 日本語
 Yorodumi
Yorodumi- PDB-1lbm: CRYSTAL STRUCTURE OF PHOSPHORIBOSYL ANTHRANILATE ISOMERASE (PRAI)... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1lbm | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF PHOSPHORIBOSYL ANTHRANILATE ISOMERASE (PRAI) IN COMPLEX WITH REDUCED 1-(O-CARBOXYPHENYLAMINO)-1-DEOXYRIBULOSE 5-PHOSPHATE (RCDRP) | ||||||
 Components Components | PHOSPHORIBOSYL ANTHRANILATE ISOMERASE | ||||||
 Keywords Keywords | ISOMERASE / BETA BARREL / ligand complex / product analogue complex / tryptophan biosynthesis | ||||||
| Function / homology |  Function and homology information Function and homology informationphosphoribosylanthranilate isomerase / phosphoribosylanthranilate isomerase activity / tryptophan biosynthetic process Similarity search - Function | ||||||
| Biological species |   Thermotoga maritima (bacteria) Thermotoga maritima (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FOURIER SYNTHESIS / Resolution: 2.8 Å FOURIER SYNTHESIS / Resolution: 2.8 Å | ||||||
 Authors Authors | Hennig, M. / Sterner, R. / Kirschner, K. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2002 Journal: Biochemistry / Year: 2002Title: Two (betaalpha)(8)-barrel enzymes of histidine and tryptophan biosynthesis have similar reaction mechanisms and common strategies for protecting their labile substrates Authors: Henn-Sax, M. / Thoma, R. / Schmidt, S. / Hennig, M. / Kirschner, K. / Sterner, R. #1:  Journal: Biochemistry / Year: 1997 Journal: Biochemistry / Year: 1997Title: Crystal structure at 2.0 A resolution of phosphoribosyl anthranilate isomerase from the hyperthermophile Thermotoga maritima: Possible determinants of protein stability | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1lbm.cif.gz 1lbm.cif.gz | 54.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1lbm.ent.gz pdb1lbm.ent.gz | 38.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1lbm.json.gz 1lbm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1lbm_validation.pdf.gz 1lbm_validation.pdf.gz | 441.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1lbm_full_validation.pdf.gz 1lbm_full_validation.pdf.gz | 445.1 KB | Display | |
| Data in XML |  1lbm_validation.xml.gz 1lbm_validation.xml.gz | 6.6 KB | Display | |
| Data in CIF |  1lbm_validation.cif.gz 1lbm_validation.cif.gz | 9.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lb/1lbm https://data.pdbj.org/pub/pdb/validation_reports/lb/1lbm ftp://data.pdbj.org/pub/pdb/validation_reports/lb/1lbm ftp://data.pdbj.org/pub/pdb/validation_reports/lb/1lbm | HTTPS FTP |
-Related structure data
| Related structure data |  1nsjS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 23070.549 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Thermotoga maritima (bacteria) / Production host: Thermotoga maritima (bacteria) / Production host:  References: UniProt: Q56320, phosphoribosylanthranilate isomerase |
|---|---|
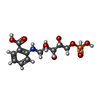
| #2: Chemical | ChemComp-137 / |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.77 Å3/Da / Density % sol: 55.64 % | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 50 mM potassium phosphate , 2.1 M ammonium sulphate, pH 7.5, VAPOR DIFFUSION, HANGING DROP, temperature 277K | ||||||||||||||||||||||||
| Crystal grow | *PLUS pH: 7 | ||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 277 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: ELLIOTT GX-20 / Wavelength: 1.5418 Å ROTATING ANODE / Type: ELLIOTT GX-20 / Wavelength: 1.5418 Å |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Jul 25, 1995 |
| Radiation | Monochromator: graphite / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→20 Å / Num. obs: 6978 / % possible obs: 99.3 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Rmerge(I) obs: 0.099 |
| Reflection | *PLUS Highest resolution: 2.8 Å / Num. measured all: 28569 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: 1nsj Resolution: 2.8→20 Å / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.8→20 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.8 Å / % reflection Rfree: 5 % / Rfactor Rwork: 0.17 | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller











 PDBj
PDBj