[English] 日本語
 Yorodumi
Yorodumi- PDB-1l5y: CRYSTAL STRUCTURE OF MG2+ / BEF3-BOUND RECEIVER DOMAIN OF SINORHI... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1l5y | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF MG2+ / BEF3-BOUND RECEIVER DOMAIN OF SINORHIZOBIUM MELILOTI DCTD | ||||||
 Components Components | C4-DICARBOXYLATE TRANSPORT TRANSCRIPTIONAL REGULATORY PROTEIN DCTD | ||||||
 Keywords Keywords | TRANSCRIPTION REGULATOR / Beryllofluoride bound two component receiver domain | ||||||
| Function / homology |  Function and homology information Function and homology informationphosphorelay signal transduction system / sequence-specific DNA binding / regulation of DNA-templated transcription / ATP hydrolysis activity / ATP binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  Sinorhizobium meliloti (bacteria) Sinorhizobium meliloti (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Park, S. / Meyer, M. / Jones, A.D. / Yennawar, H.P. / Yennawar, N.H. / Nixon, B.T. | ||||||
 Citation Citation |  Journal: FASEB J. / Year: 2002 Journal: FASEB J. / Year: 2002Title: Two-component signaling in the AAA + ATPase DctD: binding Mg2+ and BeF3- selects between alternate dimeric states of the receiver domain Authors: Park, S. / Meyer, M. / Jones, A.D. / Yennawar, H.P. / Yennawar, N.H. / Nixon, B.T. #1:  Journal: FASEB J. / Year: 2001 Journal: FASEB J. / Year: 2001Title: A dimeric two-component receiver domain inhibits the sigma54-dependent ATPase in DctD Authors: Meyer, M.G. / Park, S. / Zeringue, L. / Staley, M. / McKinstry, M. / Kaufman, R.I. / Zhang, H. / Yan, D. / Yennawar, N. / Yennawar, H. / Farber, G.K. / Nixon, B.T. | ||||||
| History |
| ||||||
| Remark 300 | BIOMOLECULE: 1 THIS ENTRY CONTAINS THE CRYSTALLOGRAPHIC ASYMMETRIC UNIT WHICH CONSISTS OF 2 CHAIN(S) ...BIOMOLECULE: 1 THIS ENTRY CONTAINS THE CRYSTALLOGRAPHIC ASYMMETRIC UNIT WHICH CONSISTS OF 2 CHAIN(S). IN THIS ENTRY CHAIN A AND B FORM THE DIMER THAT IS MOST LIKELY TO BE BIOLOGICALLY RELEVANT, BASED ON ITS USE OF THE SIGNALING SURFACE AS A DIMER INTERFACE AND UPON STRONG SIMILARITY TO FIXJ. HOWEVER, OTHER DIMER FORMS ARE PRESENT IN THE CRYSTAL LATTICE. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1l5y.cif.gz 1l5y.cif.gz | 79.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1l5y.ent.gz pdb1l5y.ent.gz | 58 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1l5y.json.gz 1l5y.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/l5/1l5y https://data.pdbj.org/pub/pdb/validation_reports/l5/1l5y ftp://data.pdbj.org/pub/pdb/validation_reports/l5/1l5y ftp://data.pdbj.org/pub/pdb/validation_reports/l5/1l5y | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1l5zC  1qkkS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | analytical ultracentrifugation indicates solution form is dimeric but which possible dimer or dimers of the crystal lattice are relevant to solution is not yet known |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 16821.326 Da / Num. of mol.: 2 / Fragment: RECEIVER DOMAIN, RESIDUES 2-143 / Mutation: E121K Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Sinorhizobium meliloti (bacteria) / Gene: dctD / Plasmid: pT143E121K / Production host: Sinorhizobium meliloti (bacteria) / Gene: dctD / Plasmid: pT143E121K / Production host:  |
|---|
-Non-polymers , 7 types, 245 molecules 


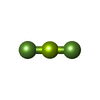
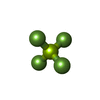




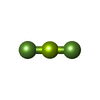



| #2: Chemical | | #3: Chemical | ChemComp-SO4 / #4: Chemical | #5: Chemical | ChemComp-BF2 / #6: Chemical | ChemComp-BF4 / | #7: Chemical | ChemComp-GOL / | #8: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.31 Å3/Da / Density % sol: 46.77 % | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7 Details: PEG 8000, lithium sulfate, HEPES, beryllium chloride, sodium fluoride, magnesium chloride, pH 7.0, VAPOR DIFFUSION, HANGING DROP, temperature 298K | |||||||||||||||||||||
| Crystal grow | *PLUS pH: 5.6 / Method: vapor diffusion, hanging drop | |||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS IV++ / Detector: IMAGE PLATE / Date: Sep 25, 2001 / Details: MSC Blue Confocal Optical System |
| Radiation | Monochromator: MSC Blue Confocal Optical System / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→43.24 Å / Num. all: 18672 / Num. obs: 18672 / % possible obs: 93.9 % / Observed criterion σ(I): 2 / Redundancy: 3.74 % / Biso Wilson estimate: 12.2 Å2 / Rmerge(I) obs: 0.105 / Net I/σ(I): 4.9 |
| Reflection shell | Resolution: 2.1→2.18 Å / Redundancy: 1.96 % / Rmerge(I) obs: 0.23 / Mean I/σ(I) obs: 2 / Num. unique all: 3331 / % possible all: 94.9 |
| Reflection | *PLUS Highest resolution: 2.1 Å / % possible obs: 99.3 % / Num. measured all: 69850 |
| Reflection shell | *PLUS % possible obs: 99.3 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1QKK Resolution: 2.1→19.87 Å / Rfactor Rfree error: 0.005 / Data cutoff high absF: 259666.55 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 58.5434 Å2 / ksol: 0.418904 e/Å3 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 18.8 Å2
| ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→19.87 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.1→2.23 Å / Rfactor Rfree error: 0.015 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS % reflection Rfree: 9.8 % | ||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller








 PDBj
PDBj









